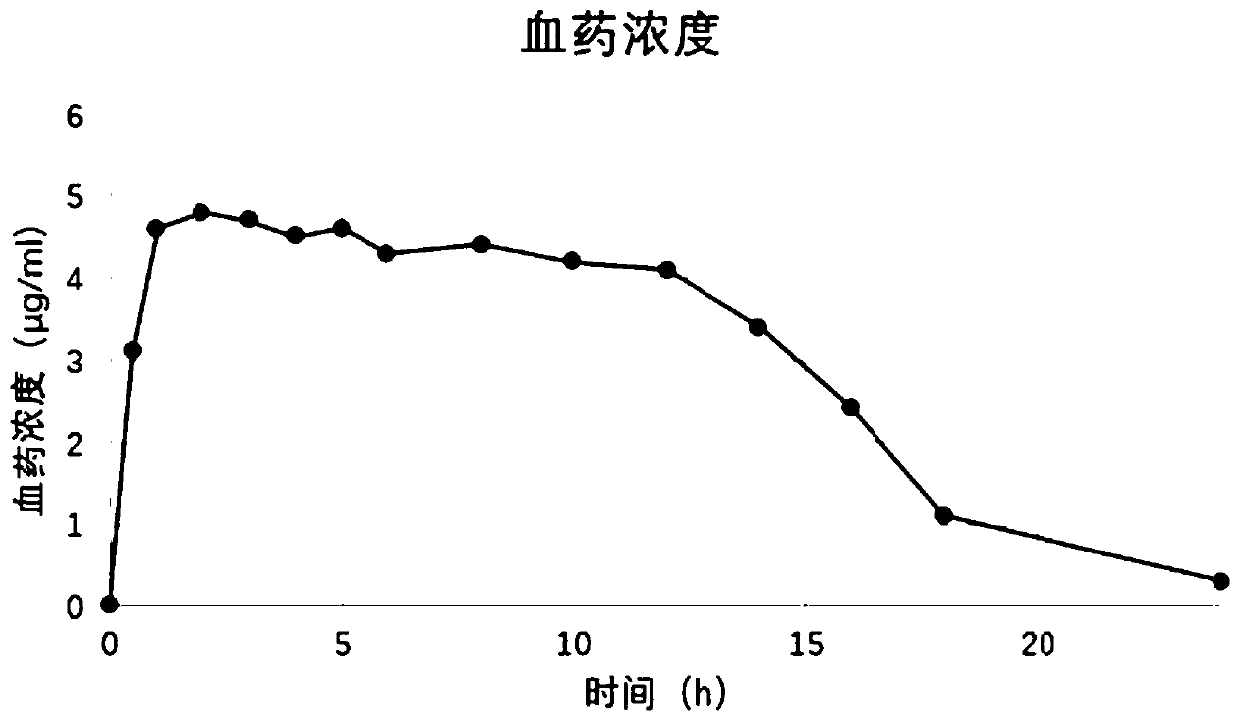
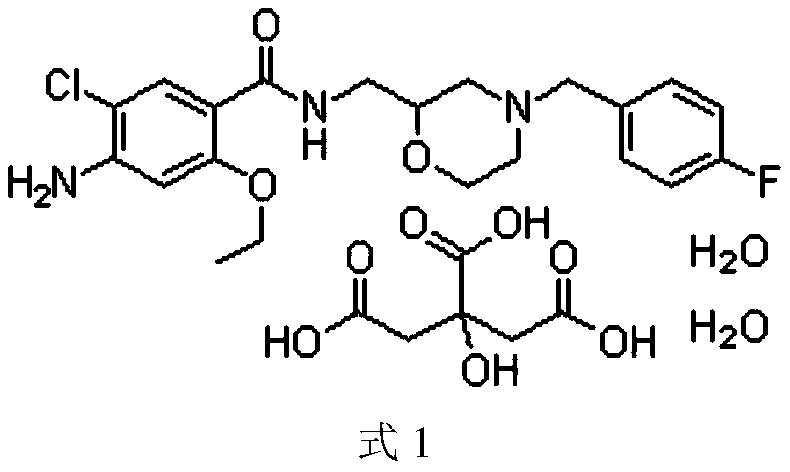
Mosapride citrate pharmaceutical composition
A technology of mosapride citrate and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of sudden release effect, unfavorable commercial production, cumbersome preparation process, etc., and achieve improved compliance and safety, and smooth release , The effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Prescription
[0033] Element proportion Mosapride Citrate 20.21% Xanthan Gum 18% polyvinyl acetate 9% Hydroxyethyl cellulose 8% Mannitol 42.79% silica 1% talcum powder 1%
[0034] (2) Preparation
[0035] Mosapride citrate is subjected to airflow pulverization treatment, polyvinyl acetate and hydroxyethyl cellulose are subjected to wet granulation and drying treatment, and the treated mosapride citrate is mixed with wet granulation dry product, yellow Raw gum and mannitol were added to the hopper mixer, mixed at 15 rpm for 40 minutes, silicon dioxide was added, mixed at 15 rpm for 10 minutes, talcum powder was added, mixed for 5 minutes, tableted according to the content controlled in the center, and coated with Opadry.
Embodiment 2
[0037] (1) Prescription
[0038]
[0039]
[0040] (2) Preparation
[0041]Mosapride citrate is subjected to airflow pulverization treatment, polyvinyl acetate and povidone are subjected to spray drying treatment, and the treated mosapride citrate is mixed with the spray-dried product, glyceryl behenate, lactose Add to the hopper mixer, mix at 15rpm for 40min, add silicon dioxide, mix at 15rpm for 10min, add sodium lauryl sulfate, mix for 5min, press the tablet according to the content controlled in the center, coat with Opadry, increase the weight by 2% .
Embodiment 3
[0043] (1) Prescription
[0044]
[0045] (2) Preparation
[0046] The mosapride citrate is jet crushed, the polyvinyl acetate and polyvinyl alcohol are spray-dried, and the treated mosapride citrate and the spray-dried product, chitosan, and lactose are added to the hopper Mixer, mix at 15rpm for 40min, add silicon dioxide, mix at 15rpm for 10min, add magnesium stearate, mix for 5min, perform tablet compression according to the content in the middle control, coat with Opadry, and increase the weight by 2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com