Triphenylamine derivative pure-organic room-temperature phosphorescent material and preparation method thereof
A technology of room temperature phosphorescence and derivatives, applied in organic chemistry methods, preparation of organic compounds, luminescent materials, etc., can solve problems such as lack of pure organic room temperature phosphorescence materials, single molecular structure, limited application research, etc., and achieve raw material price Inexpensive, simple preparation method, high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] Such as figure 1 Shown, the preparation method of above-mentioned pure organic room temperature phosphorescence material, comprises:
[0063] will react raw material And metal catalysts and alkaline reagents carry out coupling reactions in polar solvents. The reaction temperature of the coupling reaction is 10°C to 20°C higher than the boiling point of the polar solvent. The reaction time of the coupling reaction is 24-48 hours.
[0064] Wherein, alkaline reagent is potassium carbonate, alkaline reagent and reaction raw material The molar ratio is 1.2~1.5:1. Metal catalyst comprises copper powder and cuprous iodide, and the molar ratio of copper powder and cuprous iodide is 4:1, and the total amount of copper powder and cuprous iodide and reaction raw material The molar ratio is 1:4. That is to say, copper powder, cuprous iodide and reaction raw materials The molar ratio is 0.2:0.05:1. Reaction material and The molar ratio is 1:1.2~1.5. The polar solven...
Embodiment 1
[0066] Embodiment 1: the synthesis of compound A1
[0067]
[0068] Diphenylamine (846.1mg, 5.0mmol), methyl 2-iodobenzoate (1.6g, 6.0mmol), copper powder (63.6mg, 1.0mmol), cuprous iodide (47.6mg, 0.25mmol) and Potassium carbonate (829.3 g, 6.0 mmol) was dissolved in n-butyl ether (10 mL), heated to reflux under nitrogen protection, and stirred for 48 hours. After the reaction was completed, it was filtered through diatomaceous earth, washed several times with dichloromethane, the filtrate was concentrated under reduced pressure, separated by silica gel column chromatography (eluent: petroleum ether: dichloromethane = 2:1), and purified to obtain a light yellow solid product A1 (1.22 g, yield: 80.5%). Figure 22 show 1 H NMR (400MHz, CDCl 3 )δ:7.68(dd,J 1 =8.0Hz,J 2 =1.6Hz,1H),7.39-7.44(m,1H),7.15-7.22(m,6H),6.94-7.01(m,6H),3.42(s,3H).HR-MS(MALDI-TOF), m / z: calcd for C 20 h 17 NO 2 ,303.1259.Found,303.1253.
[0069] figure 2 is the overlay of the fluorescence e...
Embodiment 2
[0070] Embodiment 2: the synthesis of compound A2
[0071]
[0072] The synthetic method of compound A2 is the same as the synthetic method of A1, and the reaction substrate is aniline (931.2mg, 10.0mmol) and o-iodobenzoic acid methyl ester (5.5g, 21.0mmol), and purification obtains yellow solid product A2 (1.97g, produces rate: 54.5%). Figure 23 show 1 H NMR (400MHz, CDCl 3 )δ:7.65(dd,J 1 =8.0Hz,J 2 =1.2Hz, 2H), 7.39-7.43(m, 2H), 7.20-7.11(m, 6H), 6.86(t, J=7.2Hz, 1H), 6.76(d, J=8.0Hz, 2H), 3.39 (s,6H).HR-MS(MALDI-TOF),m / z:calcd for C 22 h 19 NO 4 ,361.1314.Found,361.1327.
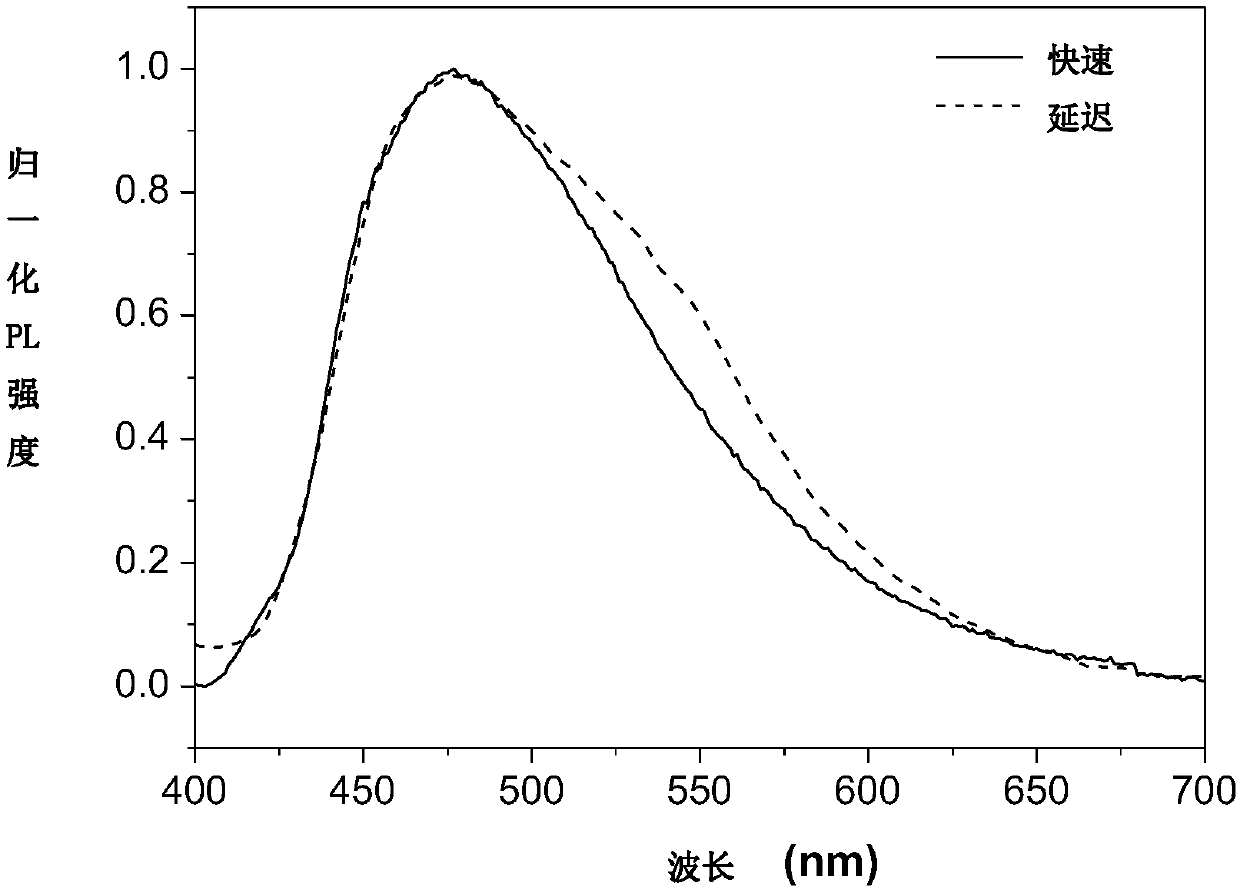
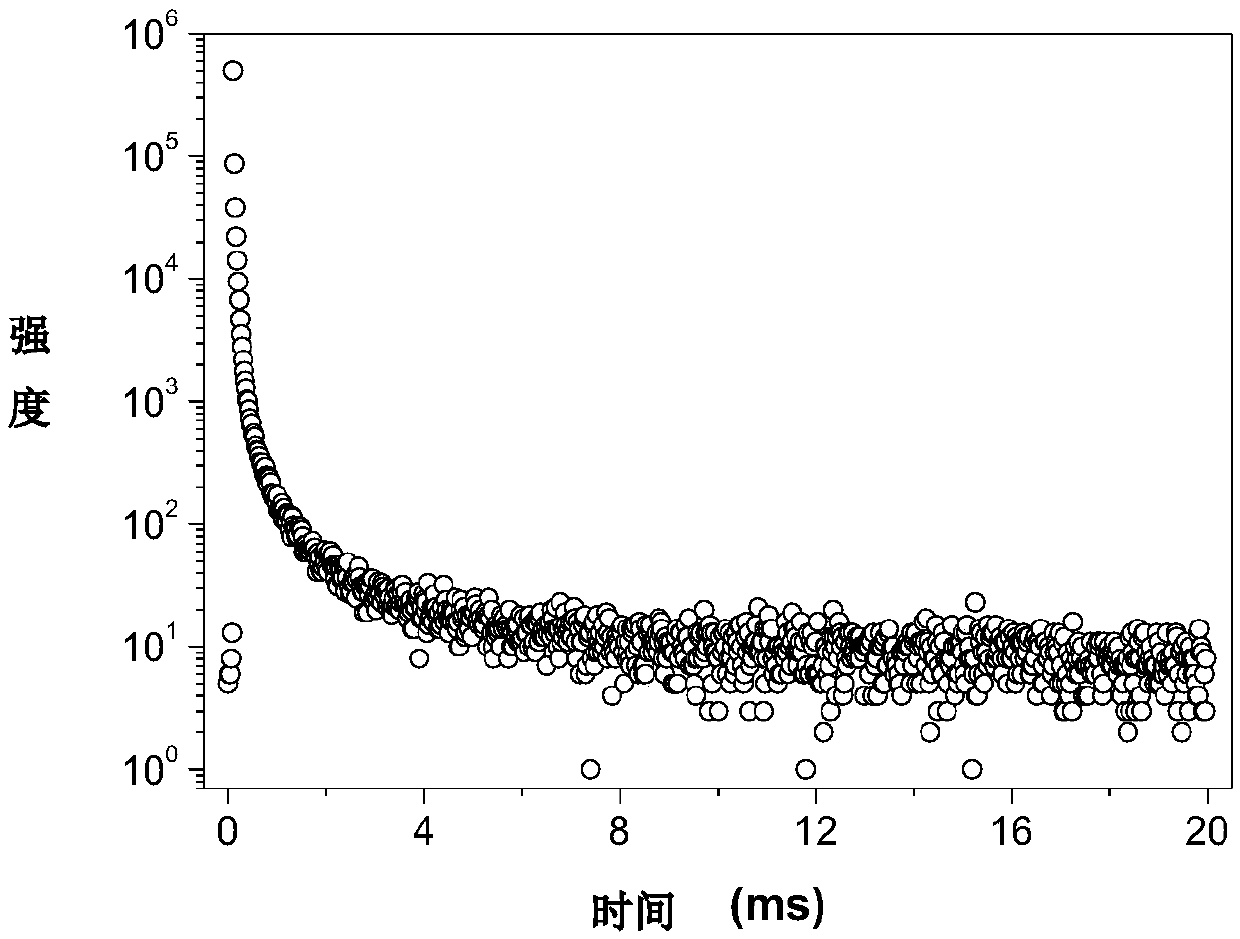
[0073] Figure 4 is the overlay of the fluorescence emission spectrum of compound A2 in the crystalline state and the steady-state emission spectrum after a delay of 1 millisecond. The figure shows that the position of the maximum emission peak on the steady-state emission spectrum after a delay of 1 millisecond has a red shift of about 42 nm relative to the fluorescence spectrum, and a new ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous life | aaaaa | aaaaa |
| Luminous life | aaaaa | aaaaa |
| Luminous life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com