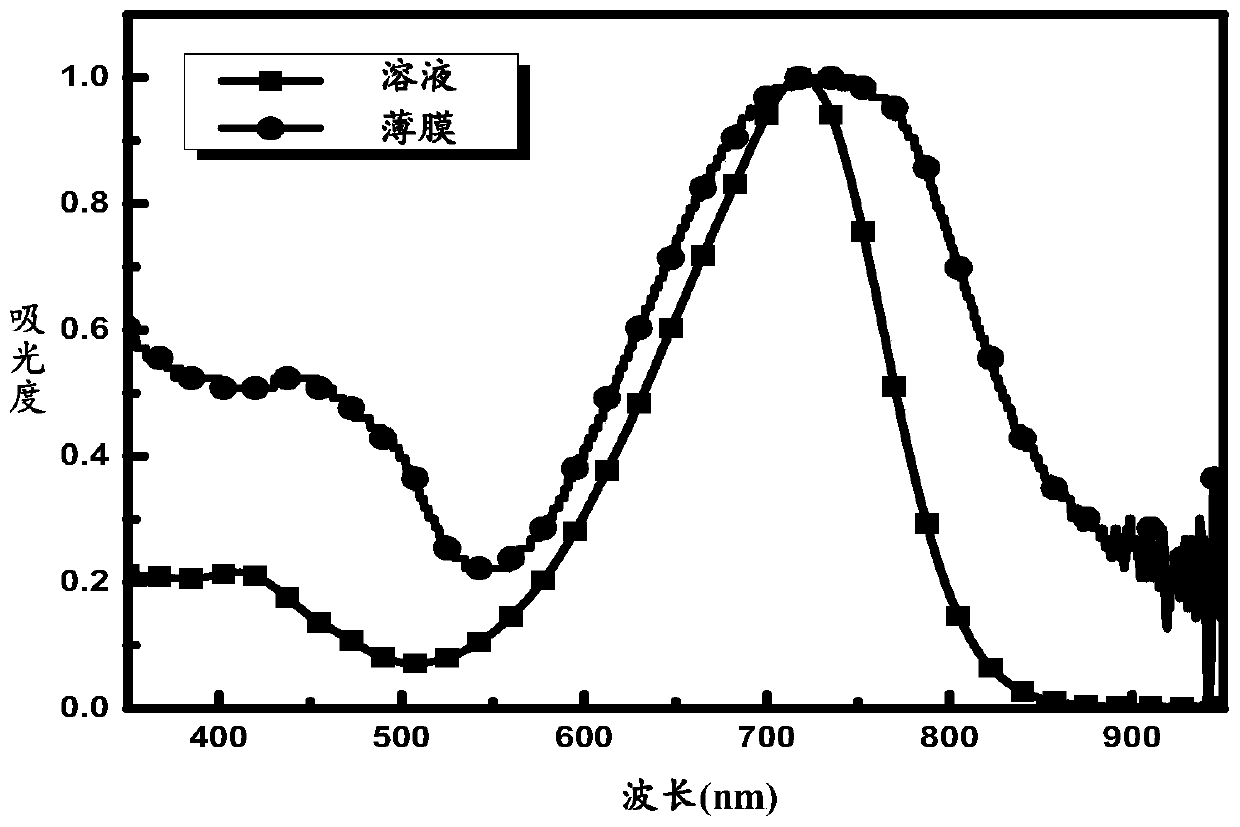
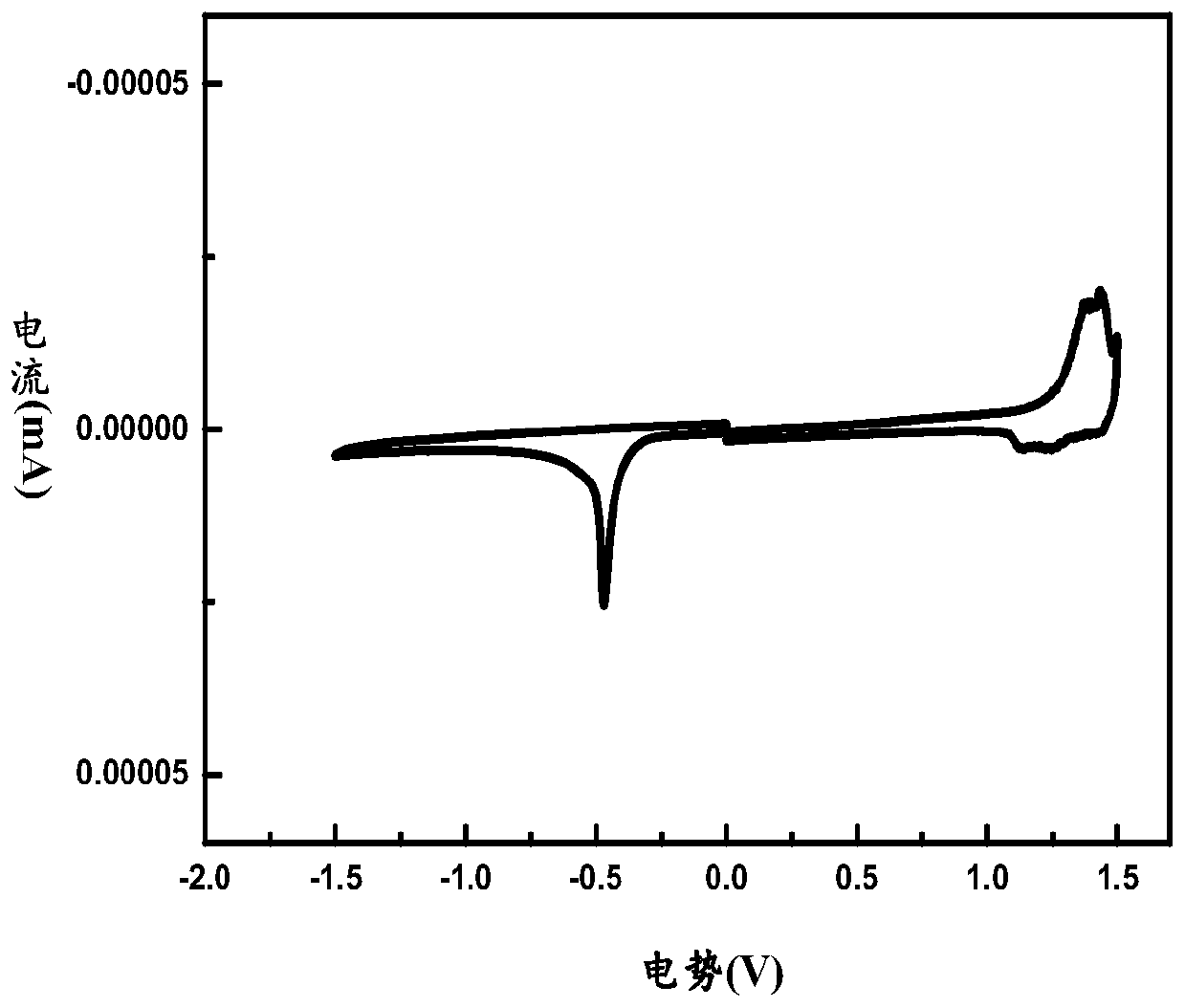
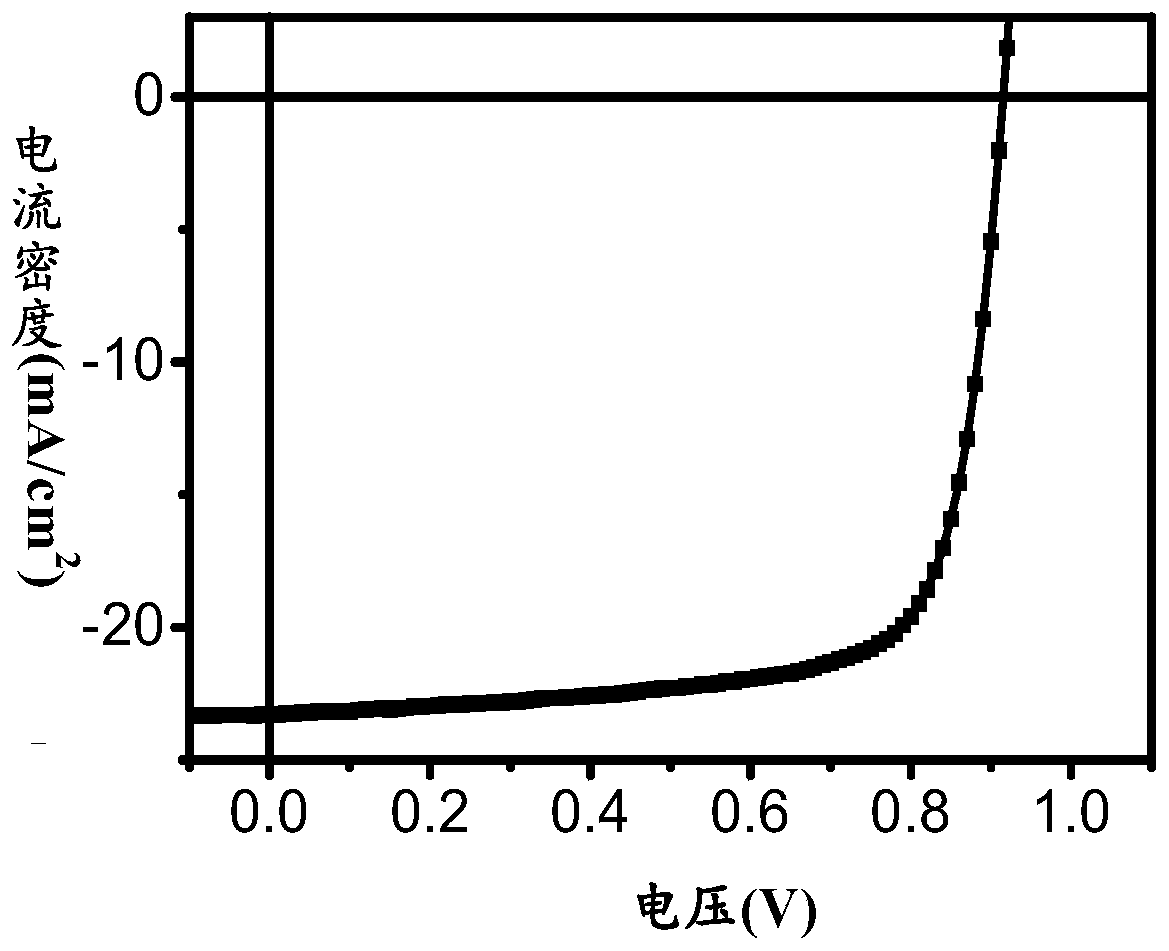
A-D-A conjugated molecule, preparation method, application in organic solar cell, and organic solar cell
An A-D-A, conjugated molecule technology, applied in organic chemistry, chemical instruments and methods, circuits, etc., can solve problems such as difficulty in adjusting absorption and energy level, no absorption of visible light, difficult purification, etc., and achieves large molar extinction coefficient and high photoelectricity. Conversion efficiency, effect of broad absorption spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] The present invention also provides a method for preparing A-D-A conjugated molecules, including the following steps:
[0078] 2,5-Dibromopyrazine and compound A are cross-coupling to obtain compound B, wherein the structural formula of compound A is The structural formula of compound B is Ar is a thiophene group, a thiophene derivative group, a dithiophene group, a dithiophene derivative group, a trithiophene group, a trithiophene derivative group, a benzodithiophene group, a benzodithiophene group Thiophene derivative group, pyrrolodithiophene group, pyrrolodithiophene derivative group, pentadithiophene group, or pentadithiophene group;
[0079] Compound B and boron tribromide undergo a condensation ring-closure reaction to obtain compound C, where the structural formula of compound C is
[0080] Compound C and dialkylaryl zinc are substituted to obtain compound D, wherein the structural formula of compound D is R 1 For 4-alkylphenyl, 4-alkoxyphenyl, 4-alkylthiophenyl, 4...
preparation Embodiment 1
[0094] The steps of preparing the A-D-A conjugated molecule of Example 1 include:
[0095] (1) 2,5-Dibromopyrazine and compound A undergo crossover (Stille) coupling reaction to obtain compound B:
[0096]
[0097] Weigh 1.0g (4.2mmol) of 2,5-dibromopyrazine and 4.1mg (10.5mmol) of compound A, put them into a 250mL round-bottomed flask, pump twice and add bis-tetratriphenylphosphine palladium Pd( PPh 3 ) 4 After repeatedly exhausting the gas in the reaction vessel and injecting inert gas to remove the air in the reaction vessel, adding 20 mL of toluene, stirring and reacting at a temperature of 110°C for 12 hours, and then cooling to room temperature; the resulting reaction liquid was rotary evaporated The solvent was removed, and the solid product obtained was separated by silica gel (200-300 mesh) column chromatography. The eluent was petroleum ether / dichloromethane (the volume ratio of the two was 3:1) to obtain a red solid ( 1.7g), which is compound B.
[0098] (2) Compound B i...
Embodiment 2
[0111] The difference between the preparation steps of the A-D-A conjugated molecule in Example 2 and the preparation step of the A-D-A conjugated molecule in Example 1 is that the end-capping group EG that reacts with compound E is different:
[0112]
[0113] Compound E (0.25g, 0.2mmol) and EG2 (0.194g, 1mmol) were sequentially added to a 50mL two-necked flask, pumped three times, and then passed N 2 Add chloroform (20 mL) in the case of, and stir for 5 min to completely dissolve the two compounds. Then pyridine (0.6 mL) was added and the solution slowly turned green. The mixture was refluxed at 65°C for 20 hours, and most of the solvent was evaporated. The crude product was washed with methanol to remove excess EG2, and was further purified by column chromatography using n-hexane: dichloromethane (2:1) as the eluent to obtain the ADA conjugated molecule of Example 2 as a dark blue solid .
[0114] The chemical structure of the ADA conjugated molecule in Example 2 was character...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Optical bandgap | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com