A kind of hyperbranched polysulfide polyamine hydrochloride and preparation method thereof
A technology of polyamine hydrochloride and amine hydrochloride, which is applied in the direction of chemicals used in biological control, antibacterial drugs, disinfectants, etc., can solve the cumbersome synthesis steps, reduce the number of effective functional groups, and limit the antibacterial effect of products Performance and other issues, to achieve the effect of fast diffusion speed and significant bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
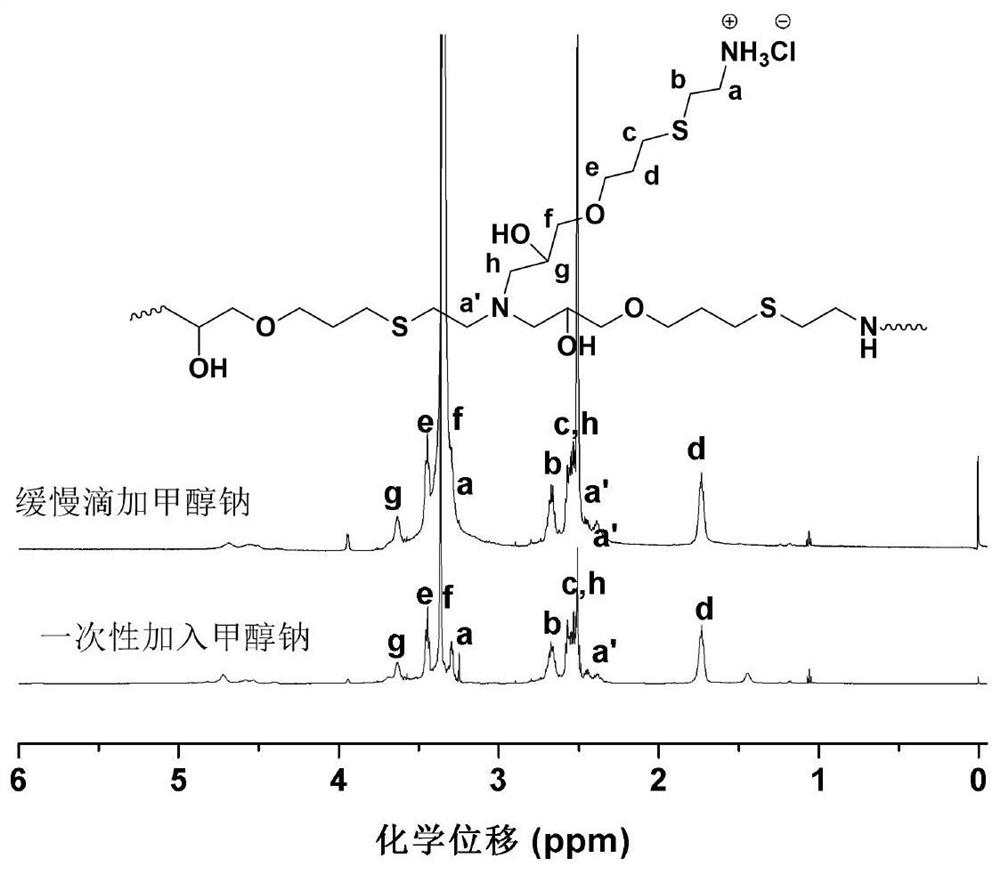
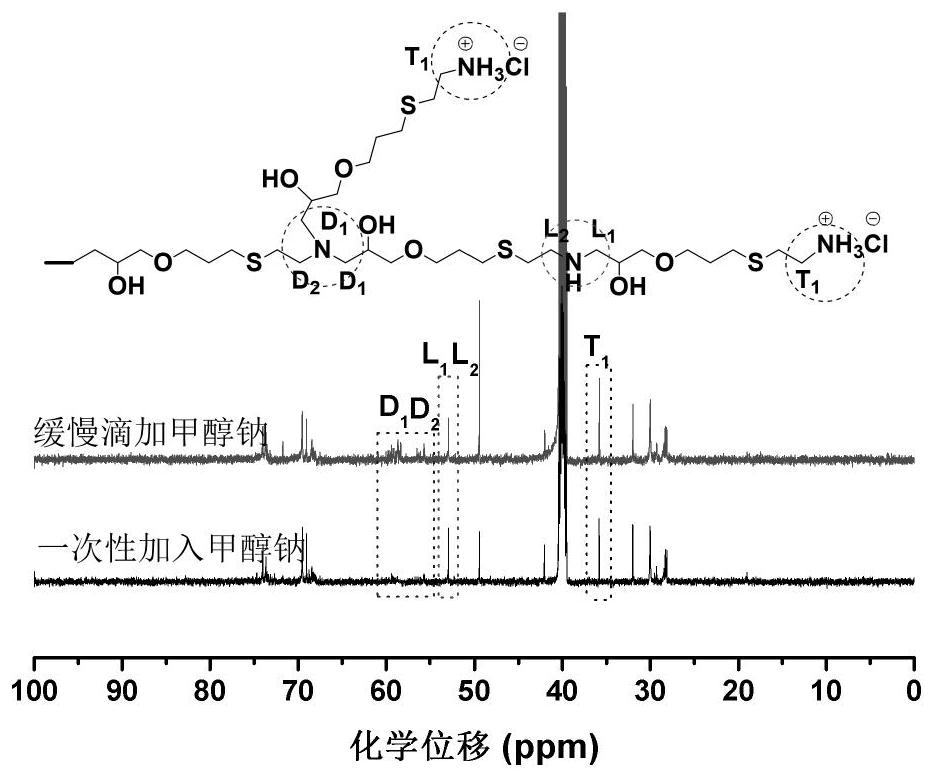
[0047] A kind of synthetic method of hyperbranched polythioether polyamine hydrochloride, one of synthetic reaction route is as follows:
[0048]
[0049]
[0050] The above is the reaction route of Example 1 taking propargyl glycidyl ether and cysteamine hydrochloride as an example for the synthesis of hyperbranched polythioether polyamine hydrochloride as starting materials, wherein, a, b, c, d, e, f is a positive integer, which is the number of molecules of the compound participating in the reaction, and the products (1)-(4) correspond to the α-epoxy-ω-amine hydrochloride intermediates of 0-3 times of epoxy-amine reaction respectively.
[0051] The specific operation steps are as follows:
[0052] S1: Add 0.336g (3mmol) of propargyl glycidyl ether, 0.681g (6mmol) of cysteamine hydrochloride, and 0.035g (0.15mmol) of benzoin dimethyl ether into a 10ml single-necked round-bottomed flask containing small magnets and 6.1 g methanol. After nitrogen protection for 30min, ...
Embodiment 2
[0065] A kind of synthetic method of hyperbranched polythioether polyamine hydrochloride, comprises the following steps:
[0066] S1: Add 0.671g (6mmol) propargyl glycidyl ether, 1.021g (9mmol) cysteamine hydrochloride, 0.014g (0.06mmol) and 15.3g methanol to a 25ml single-necked round bottom flask containing a small magnet - Water (volume ratio 9:1). At a light intensity of 6000μW / cm 2 Irradiate and react under ultraviolet light for 12 hours to obtain a light yellow transparent solution
[0067]S2: Turn on the stirring device, and raise the temperature to 60°C, and then add 0.81g (4.5mmol) sodium methoxide solution at a uniform speed within 10 hours to the α-epoxy-ω-amine hydrochloride solution obtained in step S1 (mass fraction is 30%), then continue to react for 60 hours.
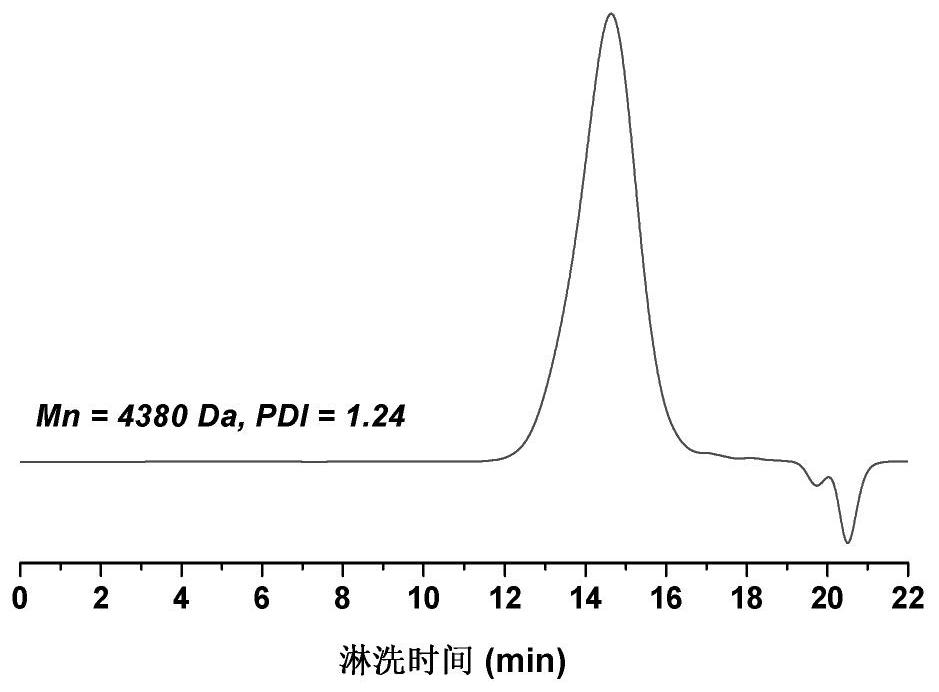
[0068] S3: After the reaction, centrifuge to remove solid sodium chloride, concentrate the supernatant with a rotary evaporator and precipitate in anhydrous ether three times, collect the precipitated...
Embodiment 3
[0072] A kind of synthetic method of hyperbranched polythioether polyamine hydrochloride, comprises the following steps:
[0073] S1: Dissolve 0.456g (4mmol) of allyl glycidyl ether, 0.409g (3.6mmol) of cysteamine hydrochloride, and 0.009 (0.04mmol) of photoinitiator α,α'-diethoxyacetophenone in 7.8g of methanol-ethanol (volume ratio 4:1) mixed solution, and then at a light intensity of 9000μW / cm 2 After 8 hours of reaction in an ice-water bath under the irradiation of ultraviolet light, a solution of α-epoxy-ω-amine hydrochloride was obtained.
[0074] S2: Turn on the stirring device, and raise the temperature to 60°C, and then add 0.335g (1.8mmol) of sodium methoxide at a uniform rate within 15 hours to the α-epoxy-ω-amine hydrochloride solution obtained in step S1 Methanol solution (mass fraction is 30%), reacted at 60°C for 48 hours.
[0075] S3: After the reaction, centrifuge to remove solid sodium chloride, concentrate the supernatant with a rotary evaporator and preci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com