A kind of preparation method of high-purity difenidol hydrochloride
A difenidol hydrochloride, high-purity technology, applied in the field of medicine, can solve problems affecting the yield of finished products, impurity content, product purity, removal of unfavorable Grignard by-product benzphenyl alcohol, and product inorganic impurities exceeding the standard, etc., to achieve crystal form Good, less impurities, fewer types of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation method of high-purity difenidol hydrochloride, concrete process is as follows:
[0034] S1. Preparation of 1-(3-chloropropyl)hexahydropyridine
[0035] Add 100g of 1,3-bromochloropropane into the reaction flask, add 54g of hexahydropyridine (1eq) dropwise under stirring at room temperature (20±5°C), and complete the dropwise addition in 2-3 hours, then react at room temperature for 2 hours. Add liquid caustic soda (77 g, 33% mass fraction) slowly, and react at room temperature for 2 hours after the addition is complete. Stop stirring, let stand for 10 to 20 hours to separate layers, and collect the upper layer oil. The oil was dried with 5 g of anhydrous sodium sulfate to obtain 97 g of 1-(3-chloropropyl)hexahydropyridine as an intermediate.
[0036] S2. Preparation of α,α-diphenyl-1-piperidinebutanol
[0037] Under a nitrogen atmosphere, add 12g of magnesium bars into the bottle, add 40g of tetrahydrofuran, 0.5g of iodine, and 0.5g of bromoethane...
Embodiment 2
[0041] A preparation method of high-purity difenidol hydrochloride, wherein the process of steps S1 and S2 is the same as in Example 1, except that the specific process of step S3 is as follows:
[0042] S3. Preparation of α, α-diphenyl-1-piperidine butanol hydrochloride
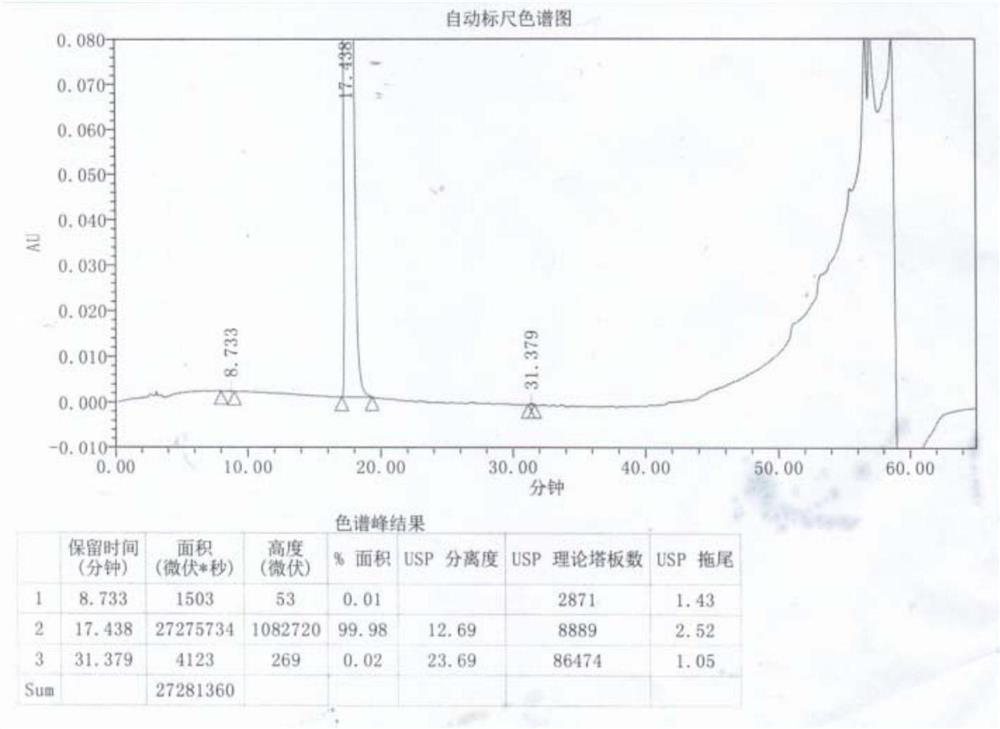
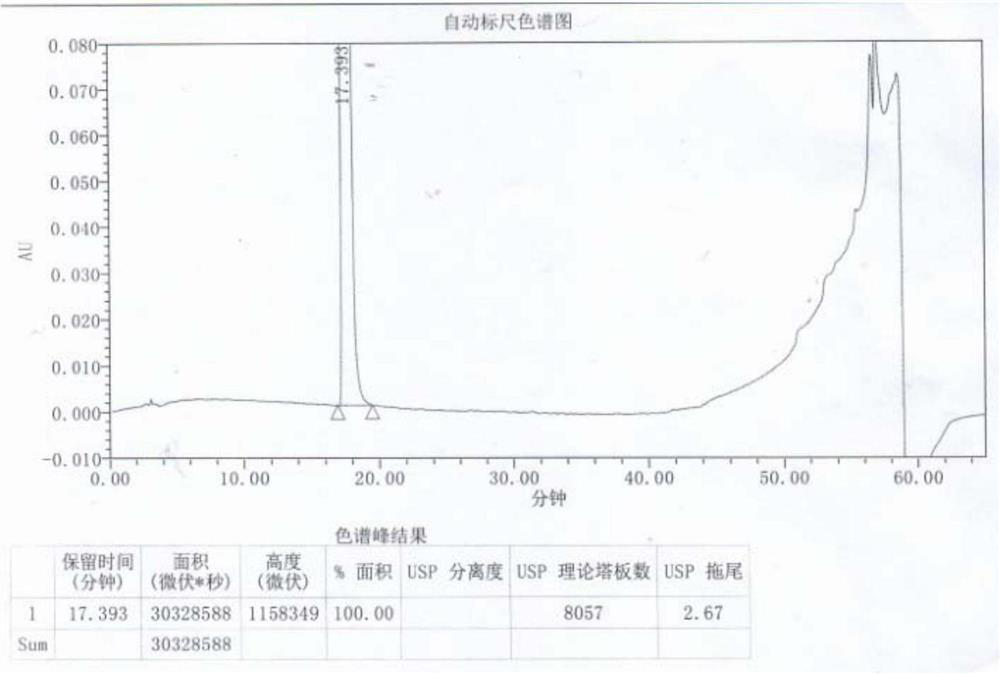
[0043] Add 8 times the mass volume of acetone to 100g of α,α-diphenyl-1-piperidinebutanol, raise the temperature to 50°C, stir to dissolve, add activated carbon and continue to stir for 0.5h, filter while hot, and filtrate at 40 Keep warm at -45°C, then add 6mol / l hydrochloric acid dropwise at a rate of 2mL / min until the pH of the solution is 6, keep stirring at 40-45°C for 1-2h, use gradient cooling, cool down to 20-25°C and stir for 1-2h , and finally lower the temperature to 5-10° C. and stir for 2-4 hours, filter and dry to obtain 103 g of off-white difenidol hydrochloride finished product, and the molar yield of salt formation is 92.14%. The finished product is tested by HPLC and has a purity of 99.95%...
Embodiment 3
[0045] A preparation method of high-purity difenidol hydrochloride, wherein the process of steps S1 and S2 is the same as in Example 1, except that the specific process of step S3 is as follows:
[0046] S3. Preparation of α, α-diphenyl-1-piperidine butanol hydrochloride
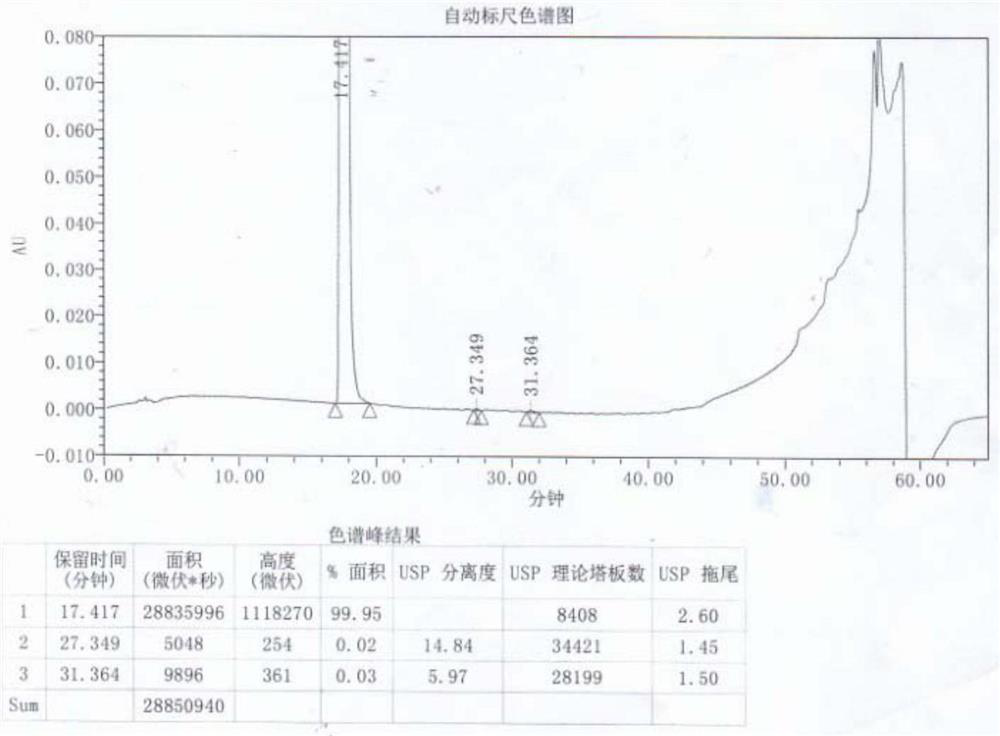
[0047] Add 6 times the mass volume of acetone to 100g of α,α-diphenyl-1-piperidinebutanol, raise the temperature to 45°C, stir to dissolve, add activated carbon and continue to stir for 0.8h, filter while it is hot, the filtrate is at 40 Keep warm at -45°C, then add 4mol / l hydrochloric acid dropwise at a rate of 5mL / min to pH 5 of the solution, keep stirring at 40-45°C for 1-2h, use gradient cooling, cool to 20-25°C and stir for 1-2h , and finally lower the temperature to 5-10° C. and stir for 2-4 hours, filter and dry to obtain 102 g of off-white difenidol hydrochloride finished product, and the molar yield of salt formation is 91.25%. The finished product is tested by HPLC, and its purity is 100.00%. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com