Preparation method of 3, 3', 4, 4'-biphenyltetracarboxylic dianhydride
A technology of biphenyltetracarboxylic dianhydride and phthalic anhydride, which is applied in the field of preparation of 3,3',4,4'-biphenyltetracarboxylic dianhydride, can solve the problem that 4-chlorophthalic anhydride is expensive and unfavorable for large-scale industrial production , serious pollution and other problems, to achieve the effect of low production cost, low pollution and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
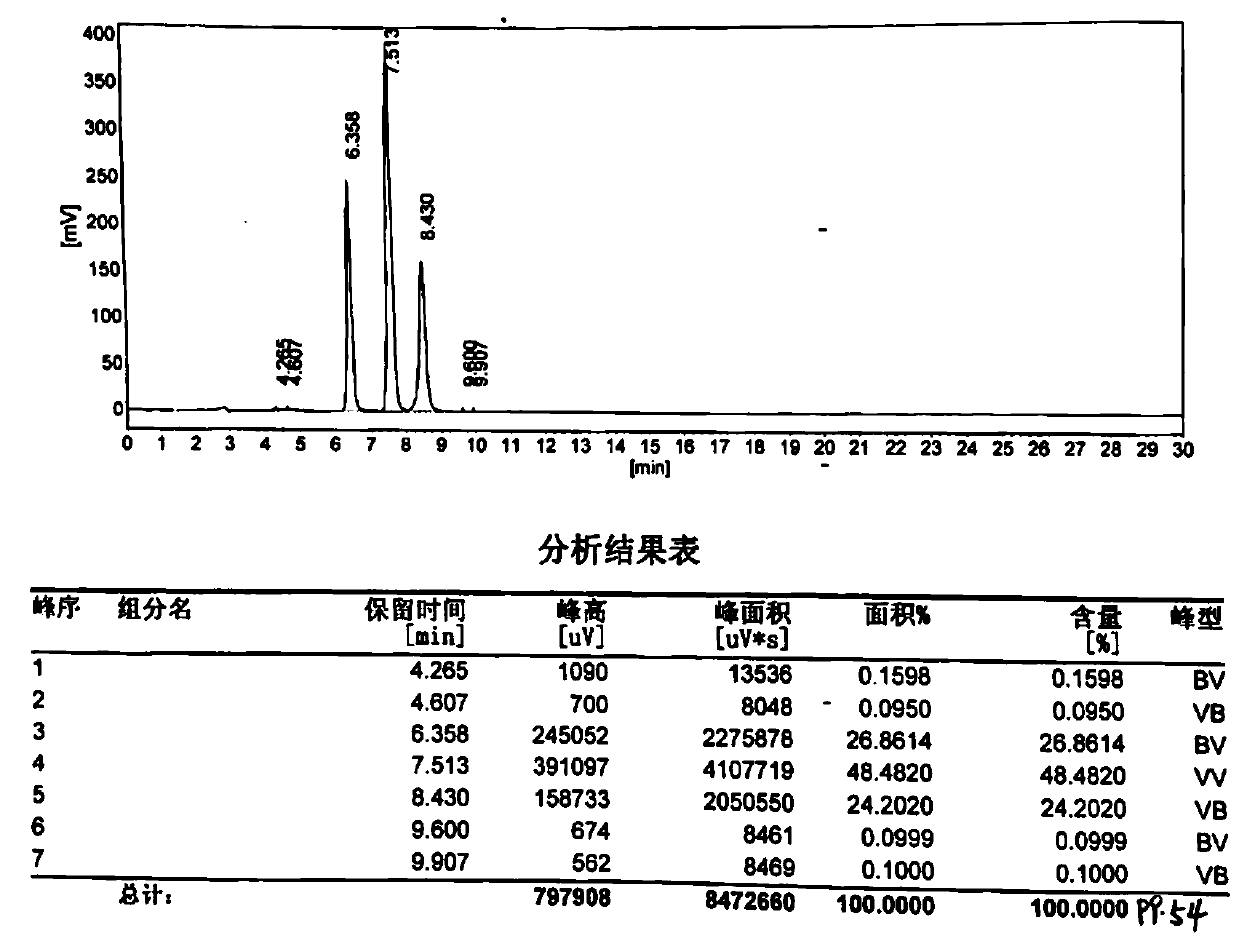
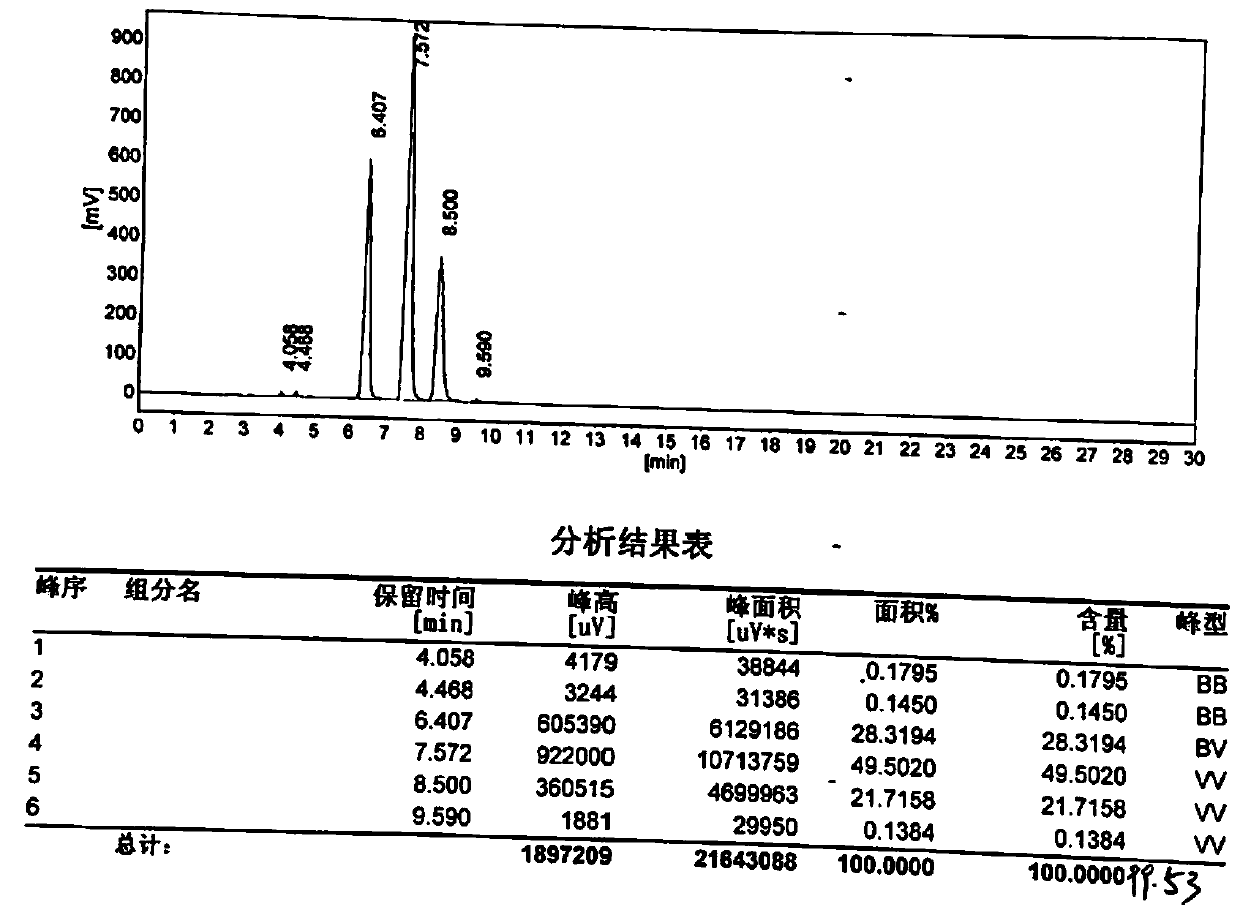
Image
Examples
Embodiment 1
[0026] Add 550g of water to a four-necked flask equipped with a thermometer, a stirrer, a dropping device and a reflux device, add 250g of phthalic anhydride under stirring, and then dropwise add 1325g of sodium hypochlorite (10% concentration, 1% free alkali (calculated as NaOH)) and 110g of liquid alkali (32% concentration). Control the dropping speed, keep the temperature at 28°C-30°C, prevent punching, and drop adding time at about 17 hours. After the dropwise addition, use about 40g of hydrochloric acid (30% concentration) to adjust the pH value to about 5.5, keep it warm at about 30°C for 0.5-1.0 hours, filter to obtain the crude product of 4-chlorophthalic acid monosodium salt, and filter cake for use.
[0027] Add 400g of water to a three-necked flask equipped with a thermometer, agitator and reflux device, add the above-mentioned filter cake material under stirring, raise the temperature to 90°C, keep it warm for 1-2 hours, then lower it to about 30°C, filter to obtai...
Embodiment 2
[0033] Add 550g of water to a four-necked flask equipped with a thermometer, a stirrer, a dropping device and a reflux device, add 250g of phthalic anhydride under stirring, and then dropwise add 1272g of sodium hypochlorite (10% concentration, 1% free alkali (calculated as NaOH)) and 85g liquid caustic soda (32% concentration) mixed solution. Control the dropping speed, keep the temperature at 28°C-30°C, prevent punching, and drop adding time for about 18 hours. After the dropwise addition, use about 40g of hydrochloric acid (30% concentration) to adjust the pH value to about 5.5, keep it warm at about 30°C for 0.5-1.0 hours, filter to obtain the crude product of 4-chlorophthalic acid monosodium salt, and filter cake for use.
[0034] Purification of the following 4-chlorophthalic acid monosodium salt, coupling into 3,3',4,4'-sodium biphenyltetracarboxylate, acidification to 3,3',4,4'-biphenyltetracarboxylic acid and It is washed with water, refined, and anhydrided at high t...
Embodiment 3
[0036] Obtain refined 4-chlorophthalic acid monosodium salt by phthalic anhydride and sodium hypochlorite with embodiment 1.
[0037] Add 300g of water and 380g of liquid caustic soda (32% concentration) into a four-necked flask equipped with a thermometer, a stirrer, a dropping device and a reflux device, and add 185g of refined 4-chlorophthalic acid monosodium salt under stirring 1. Add 1.5g palladium carbon catalyst (dry basis), heat up to reflux, drop the mixed solution of 80g hydroxylamine sulfate and 300g water, control the rate of addition, keep the liquid level stable, keep the reflux state, and the dropping time is about 6 hours. After the dropwise addition, keep warm for 1.5 hours. Cool down to 85°C and filter while hot to obtain a filtrate of sodium 3,3',4,4'-biphenyltetracarboxylate, which is set aside for use.
[0038] Add 500g of water and 175g of sulfuric acid (98% concentration) into a four-neck flask equipped with a thermometer, agitator, reflux device and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com