New use of hemin and compound thereof to medicines
A technology of hemin and compound, which is applied in the direction of medical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of joint damage prevention, high price, loss of benefit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of the stable compound of embodiment 1 Hemin
[0044] In this example, a stable complex of hemin and albumin or amino acids was prepared.
[0045] (1) Complex with albumin
[0046] Hemin with 5mg / ml Na 2 CO 3 The solution was dissolved, and the albumin was dissolved in PBS with a pH of 7.4, and an equimolar amount of albumin was added to the hemin solution, stirred while adding, and reacted overnight to obtain a hemin-albumin complex. The albumin is bovine serum albumin (bovine serum albumin, BSA) or human serum albumin (human serum albumin, HSA).
[0047] (2) Complex with amino acids
[0048] Take arginine that is 3 times the mole number of hemin and dissolve it in 10% ethanol: 40% ethylene glycol: water mixed solvent (v / v), add hemin under stirring, and react overnight to obtain the compound of hemin and arginine thing (hemin arginate). The amino acid may also be lysine or histidine.
[0049] (3) Soluble salts of hemin
[0050] Dissolve hemin i...
Embodiment 2
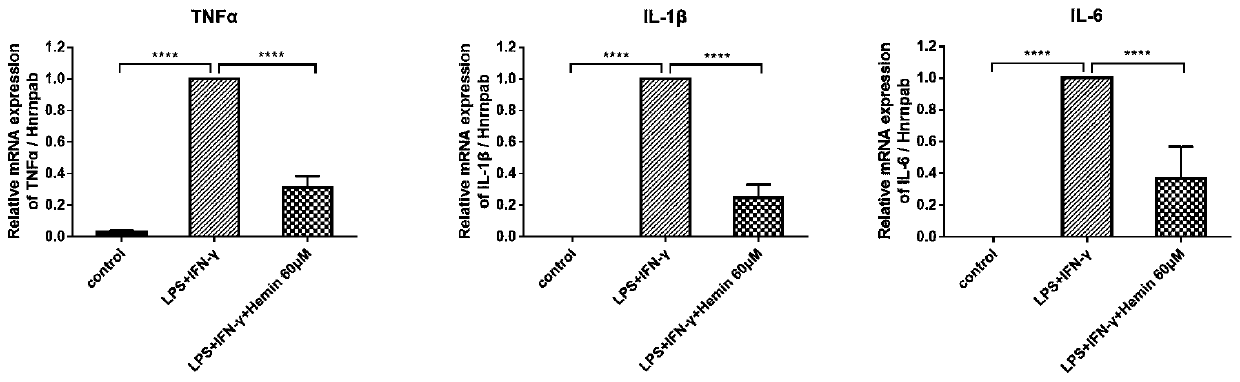
[0051] Example 2 In vitro evaluation of the anti-inflammatory effect of Hemin and the stable complex of hemin
[0052] In this example, the Raw264.7 cell inflammation model stimulated by LPS+IFN-γ was established to investigate the in vitro anti-inflammatory effect of hemin and hemin stable complex. Raw264.7 cells in the logarithmic growth phase were inoculated into 12-well plates, and after 24 hours of culture, 500ng / ml lipopolysaccharide (LPS) and 20ng / ml γ-interferon (interferon gamma, IFN-γ) were added to stimulate 6 hours to establish macrophages Inflammation model (M1 macrophage), add 1 μM, 5 μM, 10 μM, 20 μM, 30 μM, 40 μM, 50 μM, 60 μM, 80 μM, 100 μM, 150 μM, 200 μM hemin or hemin-albumin complex and hemin arginate with corresponding hemin content , hemin sodium salt, after cultivating for 16 hours, extract RNA, detect the mRNA expressions of inflammatory factors TNFα, IL-1β and IL-6 in each group by PCR, and screen the hemin concentration with the best anti-inflammator...
Embodiment 3
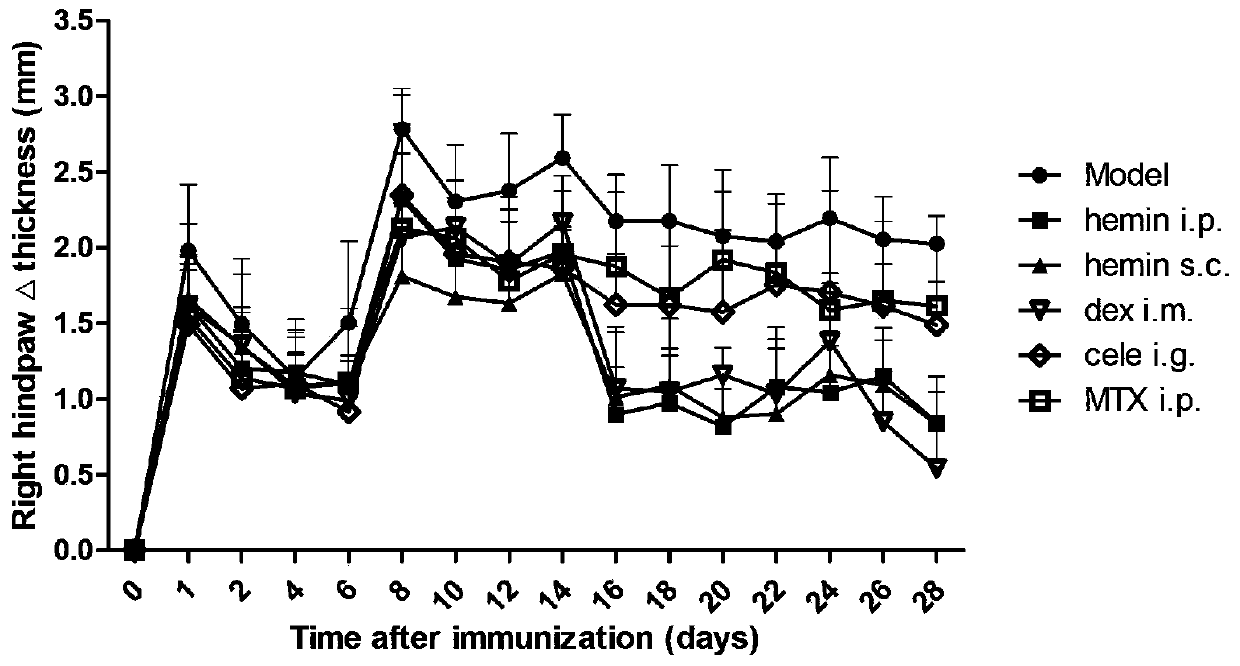
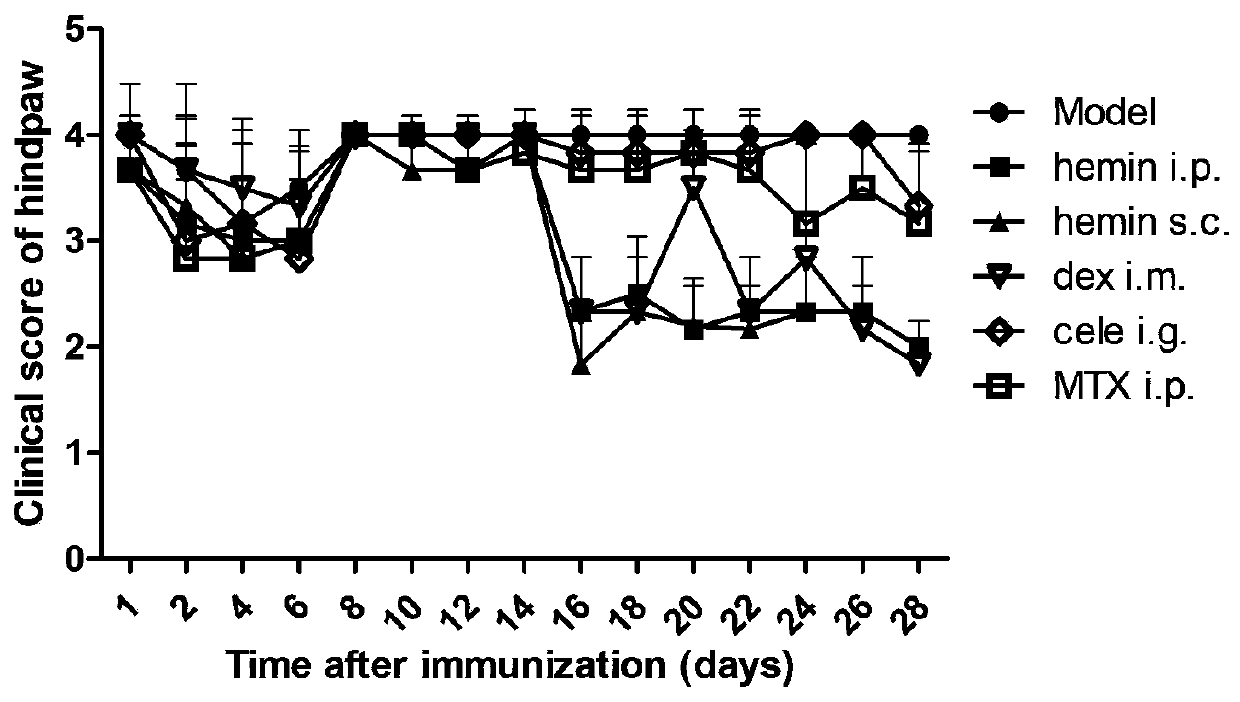
[0054] Example 3 Evaluation of anti-inflammatory effect of Hemin arginate and hemin sodium salt used for preventive administration of rat adjuvant arthritis model
[0055] In this embodiment, by establishing the rat arthritis model induced by Freund's complete adjuvant, prophylactic administration was given 1 h before the inflammation on the 0th day, and the administration was continued until 28 days after the inflammation, by measuring the hind paw thickness (mm ) and observe and evaluate the arthritis index score, and examine the preventive and therapeutic effects of hemin arginate and hemin sodium salt on rat adjuvant arthritis.
[0056] 1. Experimental animals: healthy Sprague-Dawley (SD) rats, male, weighing 250-300 g.
[0057] 2. The establishment of AIA:
[0058] Rats were injected with 0.1ml of complete Freund's adjuvant into the skin of the right hind paw of rats to induce inflammation and induce arthritis in rats.
[0059] 3. Grouping and administration of experime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com