Extraction system, extraction method and application of separating calcium and extracting lithium from calcium-containing brine with secondary amide/alkyl ketone composite solvent
A compound solvent and secondary amide technology, applied in the field of lithium extraction, can solve problems such as the limitation of the decrease in the mass ratio of calcium to lithium, the failure to realize the development of lithium resources, and the difficulty of separation and separation, so as to achieve easy control of the production process, simple structure, and easy extraction The effect of the simplicity of the separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
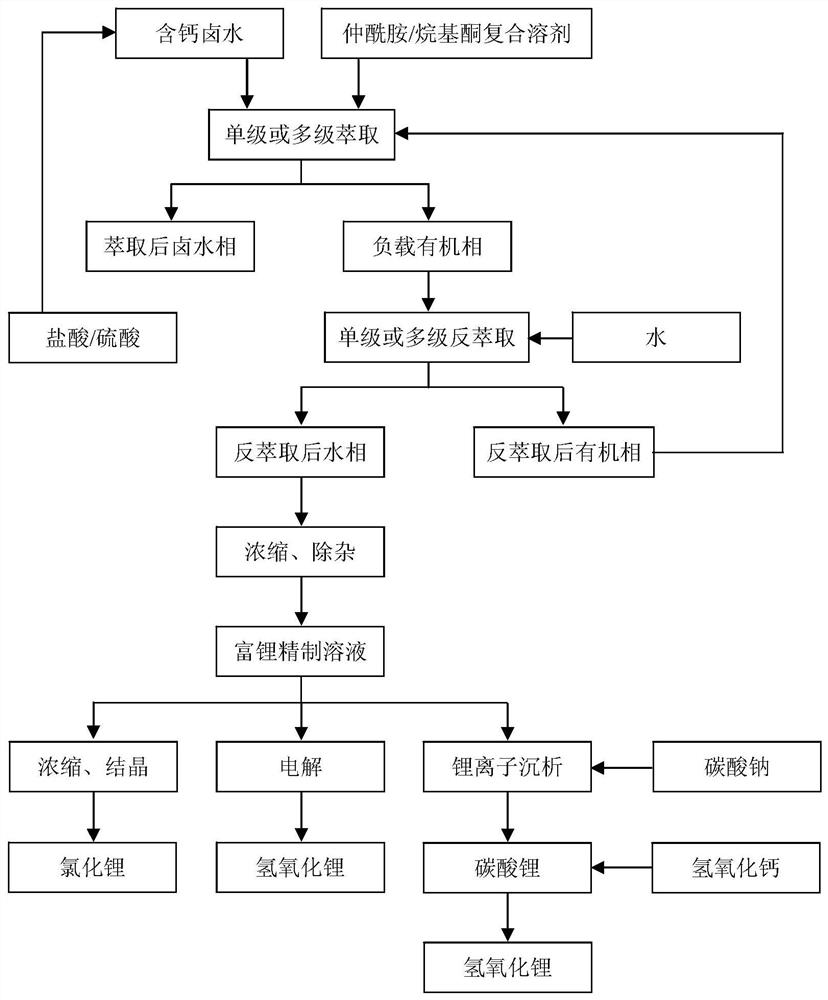
Image
Examples
Embodiment 1
[0050] Li in a calcium-containing brine + and Ca 2+ The contents are 2.92g / L and 167.29g / L respectively, and the mass ratio of calcium to lithium is 57.37:1, of which Na + 、K + , Mg 2+ , Cl - and B 2 o 3 The contents are 3.13, 20.51, 1.89, 340.05 and 2.23g / L respectively, and the brine density is 1.40g / cm 3 , the pH value of the brine is 2.1, and the ion concentration in the brine is prepared according to the brine composition of an oilfield in Nanyishan, Qaidam Basin, Qinghai. The oilfield brine is degreased by an oil-water separator in advance. Get 6mL of this kind of brine in a 100mL ground-necked Erlenmeyer flask, then add 27mL N-pentylisononamide as extractant and 3mL diisobutyl ketone as co-extractant, co-extractant occupies 10% of the organic phase volume , the volume ratio of organic phase to calcium-containing brine is 5:1. Put magnets in the Erlenmeyer flask, insert the matching air condenser into the mouth of the flask to prevent the liquid from splashing ou...
Embodiment 2
[0057] Get 27mL N-isooctyl isocaproamide as extractant and 3mL 2-nonanone as co-extractant in a 100mL ground mouth conical flask, co-extractant accounts for 10% of the organic phase volume, then add 6mL of Example 1 therein In the calcium-containing brine, the volume ratio of the organic phase to the calcium-containing brine is 5:1. Put magnets in the Erlenmeyer flask, insert the matching air condenser into the mouth of the flask to prevent the liquid from splashing out, place it in a DF-101S collector type constant temperature heating magnetic stirrer, mix and stir at 20°C, and extract for 30 minutes. Then the mixed liquid was transferred to a 100mL plastic test tube, and centrifuged in an LD5-10 desktop centrifuge at a speed of 4000r / min for 10min. The interface between the two phases was clear. After phase separation, the loaded organic phase after extraction and the remaining brine phase were obtained. Transfer the loaded organic phase to another 100mL ground-mouth Erlenme...
Embodiment 3
[0063]Take 16mL N-isooctyl isovaleramide as extractant and 16mL methyl nonyl ketone as co-extractant in a 100mL ground-necked Erlenmeyer flask, co-extractant accounts for 50% of the volume of the organic phase, and then add 4mL For the calcium-containing brine in Example 1, the volume ratio of the organic phase to the calcium-containing brine is 8:1. Put magnets in the Erlenmeyer flask, insert the matching air condenser into the mouth of the flask to prevent the liquid from splashing out, place it in a DF-101S collector type constant temperature heating magnetic stirrer, mix and stir at 0°C, and extract for 30 minutes. Then the mixed liquid was transferred to a 100mL plastic test tube, and centrifuged in an LD5-10 desktop centrifuge at a speed of 4000r / min for 10min. The interface between the two phases was clear. After phase separation, the loaded organic phase after extraction and the remaining brine phase were obtained. Transfer the loaded organic phase to another 100mL gro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com