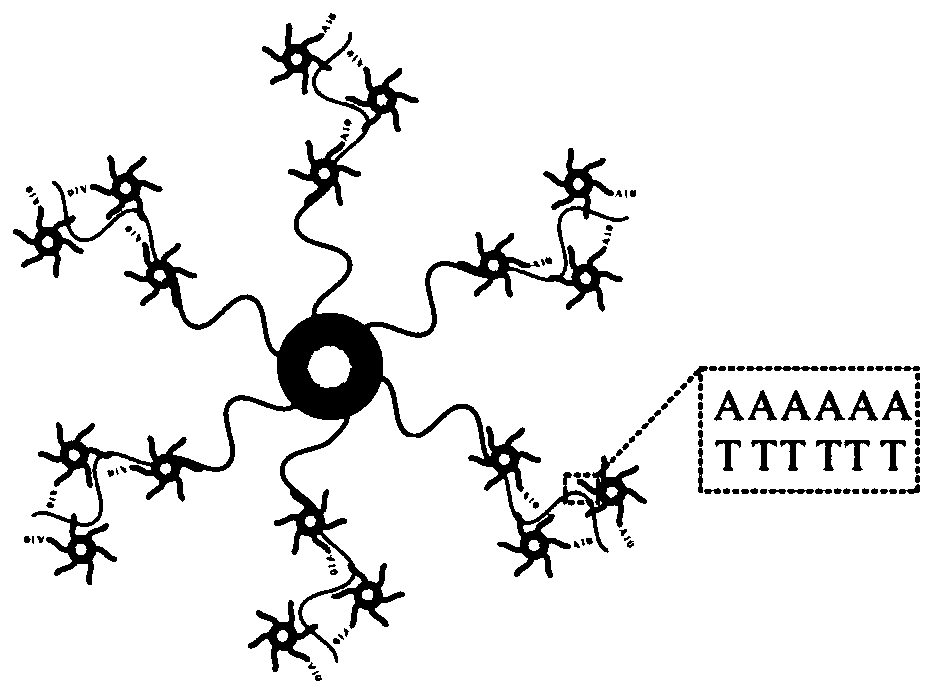
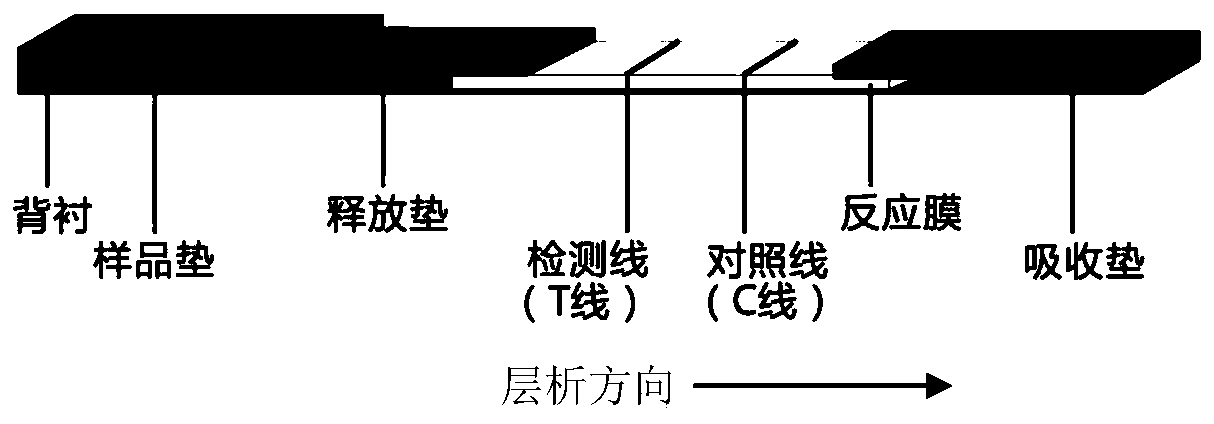
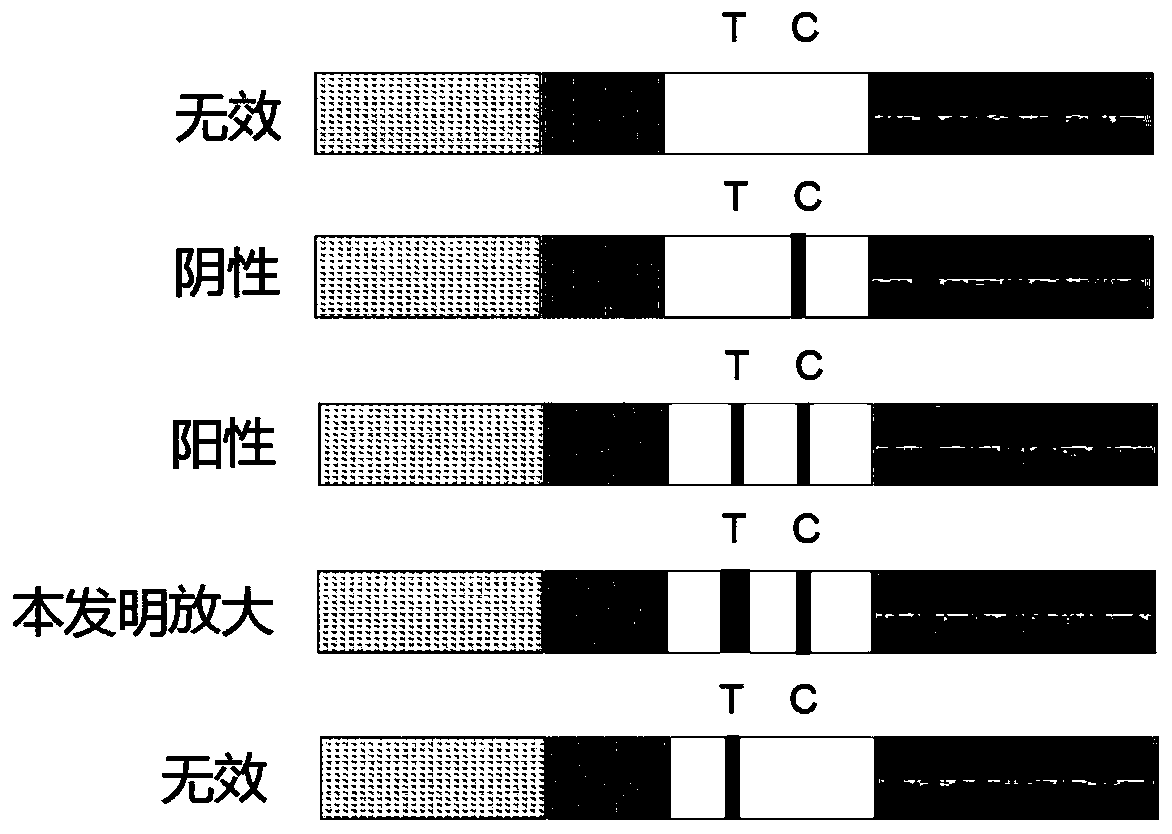
Test strip based on TdT signal amplification technique and preparation method thereof
A technology of signal amplification and test strips, applied in biological testing, instruments, measuring devices, etc., can solve the problems of low sensitivity and difficult to achieve rapid target analysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation and detection of colloidal gold test strips for the detection of enrofloxacin
[0065] (1) Preparation of colloidal gold
[0066] After 99g of water and 1mL of 10g / L chloroauric acid solution were magnetically stirred and heated to boiling in an oil bath, 3.5mL of 10g / L sodium citrate solution was added to react for 20 minutes, and then the heating was stopped. Stir vigorously and then overnight to obtain colloidal gold, colloidal gold The particle size of the gold nanoparticles was 15 nm and was stored at 4°C for later use.
[0067] (2) Assembly of gold nanoparticles and enrofloxacin aptamer 1 (aptamer1)
[0068] Mix 20 μL of sulfhydryl-modified enrofloxacin aptamer 1 (aptamer1, 100 μmol) with 1 mL of colloidal gold (2.3 nM). After overnight at 25°C, add 113 μL of 0.1 M PB (the final concentration of PB is 0.01 M), and shake at room temperature for 30 min. ; Add PB solution (PB concentration of 0.01M) containing sodium chloride (2M) with a total...
Embodiment 2
[0097] Embodiment 2 Preparation and detection of colloidal gold test strips for potassium ion detection
[0098] (1) Preparation of gold sol: the preparation process is the same as that in Example 1.
[0099] (2) Assembly of gold nanoparticles and potassium aptamer 1: the preparation process is the same as that of Example 1, except that the DNA sequence of potassium aptamer 1 (aptamer1) is: 5'-SH-AAAAAAAAAAGGTTGGT-Biotin-3 ', 5' modified sulfhydryl group.
[0100] (3) Amplification of Au-aptamer1: the preparation process is the same as that of Example 1.
[0101] (4) Preparation of dendritic nano-gold composite solution: the preparation process is the same as that in Example 1.
[0102] (5) Preparation of binding release pad: the preparation process is the same as that of Example 1.
[0103] (6) The preparation process of the incubation solution of streptavidin-biotin-labeled potassium aptamer 2 (aptamer2) is the same as that in Example 1, except that the DNA sequence of po...
Embodiment 3
[0108] Example 3 Preparation and detection of colloidal gold test strips for kanamycin detection
[0109] (1) Preparation of gold sol: the preparation process is the same as that in Example 1.
[0110] (2) Assembly of gold nanoparticles and potassium ion aptamer 1: the preparation process is the same as that of Example 1, except that the DNA sequence of kanamycin aptamer 1 (aptamer1) is: 5'-SH-AAAAAAAAAATGGGGGTTGAG-3 '.
[0111] (3) Amplification of Au-aptamer1: the preparation process is the same as that of Example 1.
[0112] (4) Preparation of dendritic nano-gold composite solution: the preparation process is the same as that in Example 1.
[0113] (5) Preparation of binding release pad: the preparation process is the same as that of Example 1.
[0114] (6) The preparation process of the incubation solution of streptavidin-biotin-labeled kanamycin aptamer 2 (aptamer2) is the same as that in Example 1, except that: kanamycin aptamer 2 (aptamer2) The DNA sequence is: 5'-GCT...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com