Synthesis and application of novel triazine-derivative fire retardant
A triazine derivative, a new type of technology, applied in the direction of flame-retardant fibers, plant fibers, and compounds of Group 5/15 elements of the periodic table, etc., can solve application limitations, affect material mechanical properties, human health and environmental hazards, etc. problem, to achieve the effect of good charcoal performance, good thermal performance and good flame retardant performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
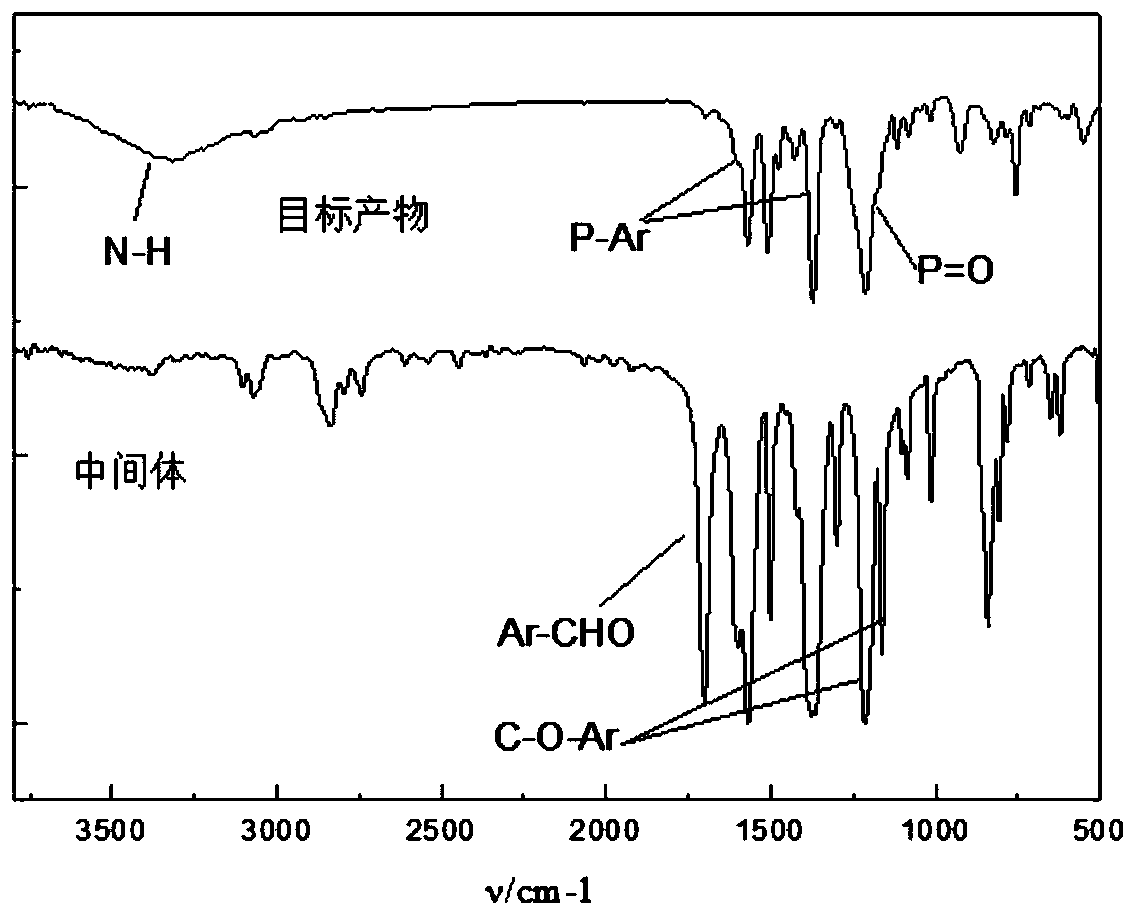
[0042] Synthesis of 2,4,6-tris(4-formylphenoxy)-1,3,5-triazine (ZJT).
[0043] Weigh 22.00g (0.054mol) of p-hydroxybenzaldehyde, 10.00g (0.18mol) of cyanuric chloride in a 100mL beaker, add 80.00mL of dichloromethane to dissolve, 0 ~ 5 ℃ ice water bath, stir, add 6.00mL of triethyl Amines (TEA) were used as phase transfer catalysts. Weigh 7.20g of sodium hydroxide and dissolve in 50.00mL of distilled water. Then sodium hydroxide solution was added slowly and stirred for 10 minutes to complete the reaction. The above-mentioned reactants were introduced into a 500 mL three-necked flask, and heated to reflux for two hours. Then dichloromethane was distilled off, filtered, dried, and recrystallized from ethyl acetate. The organic intermediate 2,4,6-tris(4-formylphenoxy)-1,3,5-triazine (ZJT) is obtained. Yield: 95.86%. FTIR (KBr), ν / cm-1: 1161.95, 1214.49 (C-O-Ar), 1569.18, 1501.37, 1376.00 (Ar), 2836.37, 2742.30, 1701.16 (Ar-CHO). 1 H NMR (CDCl3, 300 MHz) δ: 7.31-7.33 (t, 6H...
Embodiment 2
[0045] Synthesis of Novel Triazine Derivative Flame Retardant Material NPFR
[0046]Weigh 7.58g (69mmol) of p-aminophenol into 150.00mL of 1,4-dioxane, heat until completely dissolved, then add 9.35g (21mmol) of the intermediate, and heat to reflux for 12h under nitrogen protection. Weigh 14.60g DOPO (69mmol) and dissolve it in 60.00mL 1,4-dioxane, heat until completely dissolved, and the solution is colorless and transparent. Then DOPO solvent was slowly added to the above reaction, vacuumed, heated to reflux under nitrogen protection for 12 hours, rotary evaporated, washed with cold ethanol, and dried in a vacuum oven at 60°C for 24 hours. The yield was 94.76%. FTIR(KBr), v / cm -1 : 3317.65 (N-H), 1569.37, 1371.31 (P-Ar), 1213.11 (P=O). 1 H NMR (DMSO-d6, 300MHz) δ: 8.46-8.44 (m, 3H), δ: 8.20-7.02 (m, 36H), δ: 6.54-6.19 (m, 12H), 6: 5.68-5.58 (m, 3H), δ: 5.07-5.01 (m, 3H).
Embodiment 3
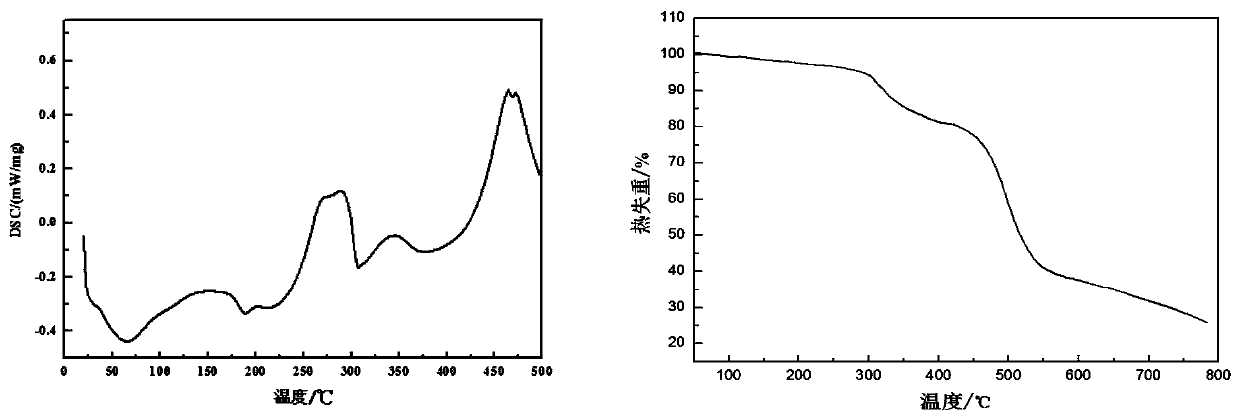
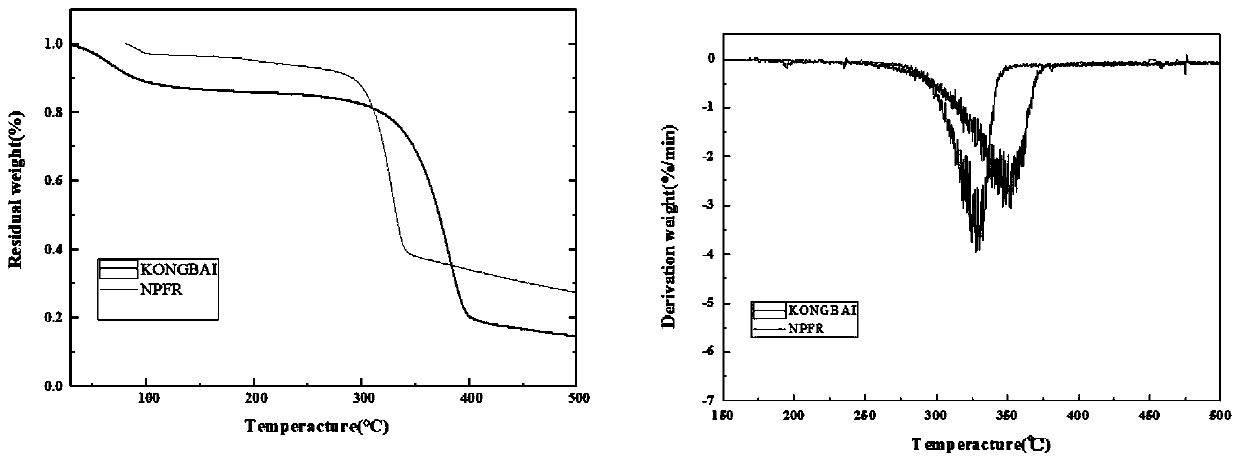
[0048] The thermodynamic analysis of embodiment 2 gained novel triazine derivative flame retardant material (NPFR)
[0049] The test conditions of DSC thermodynamic analysis of NPFR are: in N 2 In the atmosphere, the temperature rises from room temperature to 280°C at a rate of 10°C / min, and the TG analysis test conditions are: in N 2 In the atmosphere, the temperature was raised from room temperature to 700°C at a rate of 20°C / min. The DSC and TG analysis results of NPFR were as follows: figure 1 shown. In the DSC curve of NPFR, 189.5°C is the absorption peak when NPFR melts, and the phosphorus-containing DOPO group of NPFR-2 begins to decompose when heated at 261.1°C. As the temperature rises, absorption peaks appear successively at 324.2°C, 349.6°C, 387.1°C, 462.1°C, etc., indicating that other bonds such as triazine ring and benzene ring are broken successively. The TG test results of NPFR are basically consistent with DSC. As the temperature rises, around 260-420°C is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com