An analysis method for measuring related substances in diclofenac sodium raw materials and preparations thereof
A technology for diclofenac sodium and related substances is applied in the analysis field of measuring diclofenac sodium raw materials and related substances in preparations thereof, and can solve the problems of inability to accurately and effectively measure the content of impurity D, affecting the recovery rate of impurity D, poor separation of diclofenac sodium, and the like, Achieve reliable quality control methods, improved recovery, and improved resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] UPLC conditions:
[0039] The chromatographic column is octadecylsilane-bonded silica gel column YMC-Triart (2.0mm×100mm, 1.9um), with acetonitrile as mobile phase A, and 0.7708g / L ammonium acetate solution (adjusted to pH 5.3 with acetic acid) as mobile phase In phase B, the detection wavelength is 254 nm, the flow rate is 0.3 ml / min, and the column temperature is 35 °C, and gradient elution is performed. The gradient elution procedure is shown in Table 2.
[0040] Table 2 Gradient elution program table
[0041]
[0042] Sample preparation:
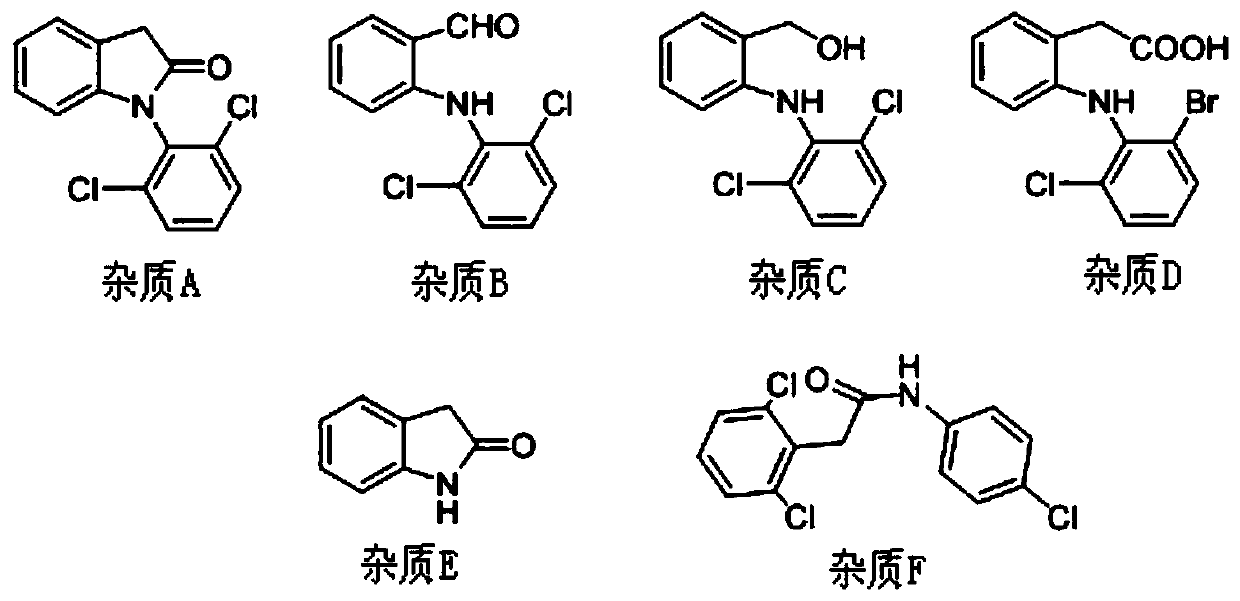
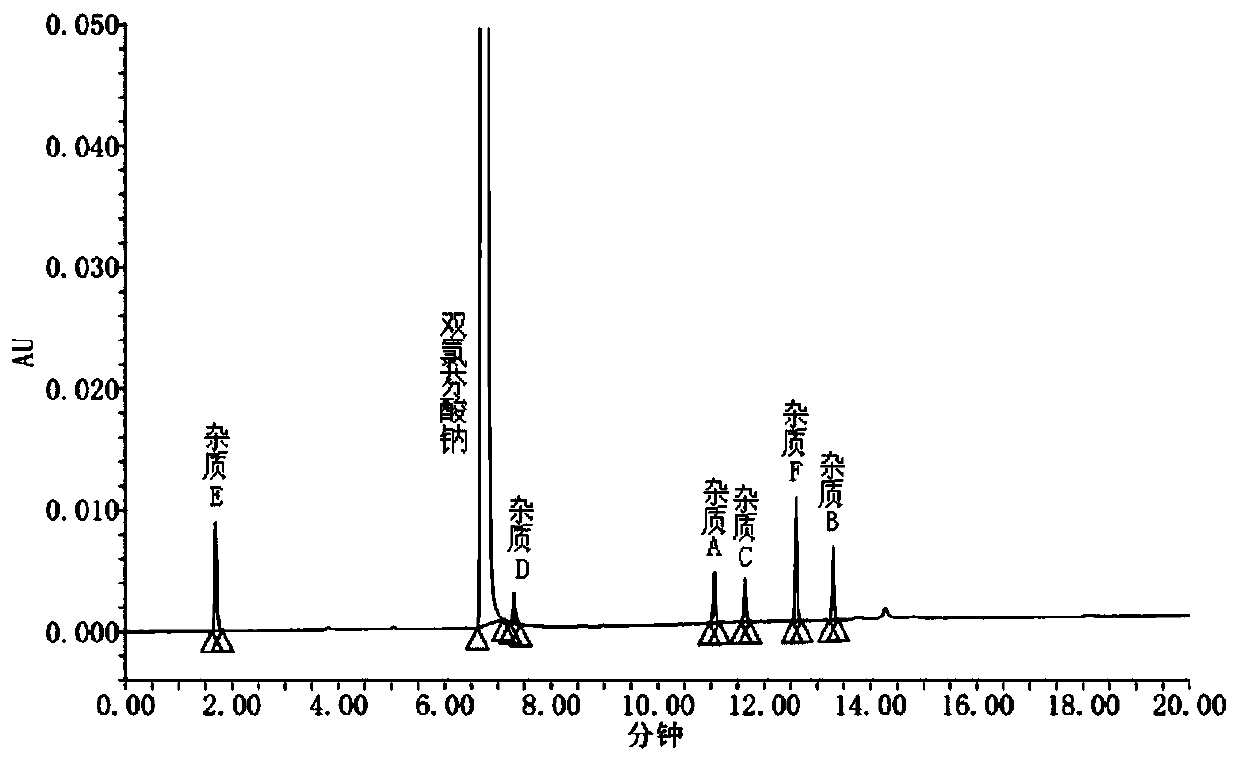
[0043] System suitability solution: Weigh appropriate amounts of diclofenac sodium reference substance and impurity A, B, C, D, E, F reference substance respectively, accurately weigh, dissolve and dilute with 50% acetonitrile to prepare each 1ml containing approximately diclofenac The mixed solution of 1 mg of sodium and impurities A, B, C, D, E, and F is 2 μg as the system suitability solution.
[0044] Test solution: take...
Embodiment 2
[0049]Ultra-high performance liquid chromatography conditions: the same as in Example 1.
[0050] Sample preparation:
[0051] System suitability solution: same as Example 1.
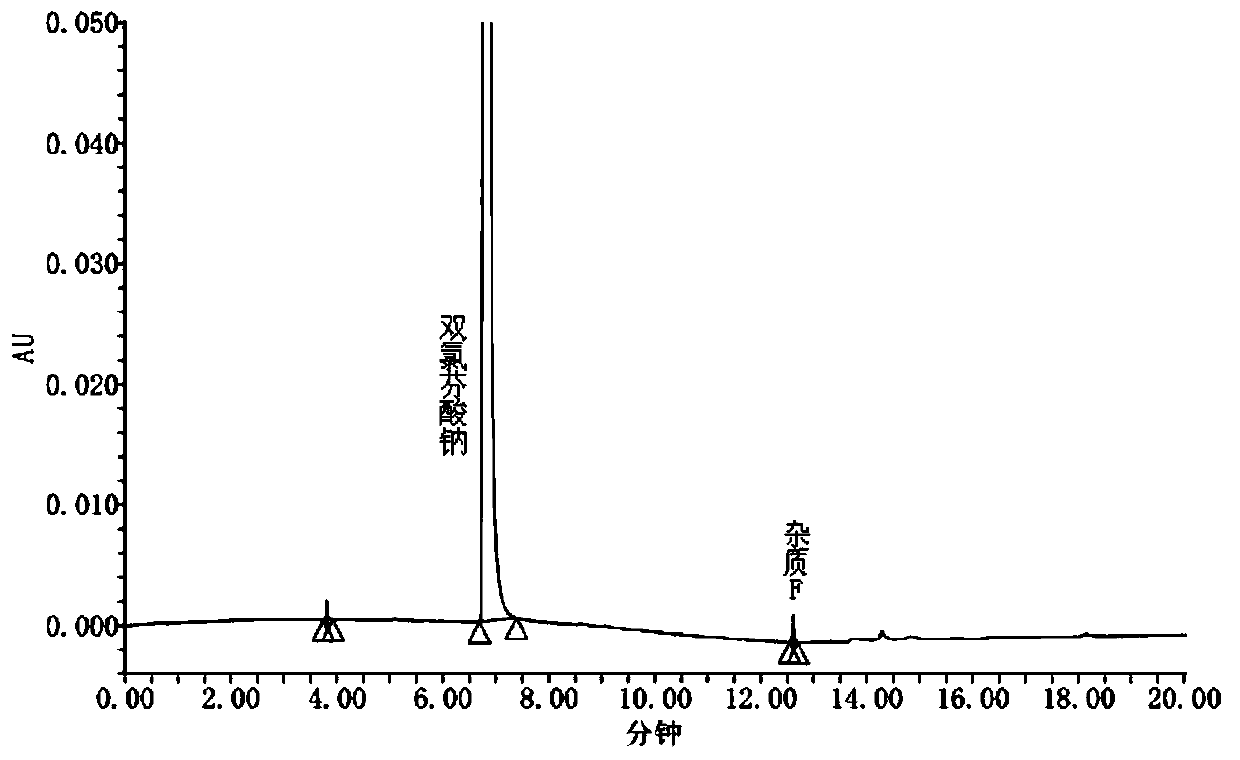
[0052] Test solution: Take 20 diclofenac sodium sustained-release tablets (specification: 100mg), grind and mix well, take an appropriate amount of fine powder (equivalent to about 50mg of diclofenac sodium), accurately weigh it, put it in a 50ml measuring bottle, add 50% acetonitrile About 35ml, ultrasonic for 25min, dilute to the mark with 50% acetonitrile, shake well, centrifuge, take the supernatant and filter it with 0.45μm filter membrane, take the filtrate as the test solution.
[0053] Reference substance solution: Take an appropriate amount of diclofenac sodium reference substance, accurately weigh it, dissolve it with 50% acetonitrile and quantitatively dilute it to make a solution containing about 1 μg of diclofenac sodium per 1 ml, as the reference substance solution.
[0054] Determinatio...
Embodiment 3
[0057] Ultra-high performance liquid chromatography conditions: the same as in Example 1.
[0058] Sample preparation:
[0059] Precisely weigh impurity A 10.06 mg, impurity B 10.53 mg, impurity C 10.31 mg, impurity D 9.65 mg, impurity E 10.20 mg, impurity F 9.85 mg, and diclofenac sodium 10.01 mg, respectively, put them in 25ml measuring flasks, dissolve 50% acetonitrile, Dilute to the mark, shake well, and use it as the stock solution for each reference substance. Accurately measure an appropriate amount of each reference substance stock solution, dilute it with 50% acetonitrile to prepare a series of solutions, and precisely measure 1 μl according to the chromatographic conditions of Example 1, inject it into a liquid chromatograph, and record the chromatogram. Taking the concentration (μg / ml) as the X-axis and the peak area as the Y-axis, the linear regression equation and correction factor (ratio of the slope of diclofenac sodium to the slope of each impurity) of each co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Filler particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com