Cobaltosic oxide catalyst, preparation method and application thereof
A technology of cobalt tetroxide catalyst and cobalt tetroxide, which is applied in catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve problems such as high cost and easy deactivation, and achieve simple raw materials. , The effect of lowering the active energy barrier and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of tricobalt tetroxide catalyst of the present invention comprises:
[0035] (a) Preparation of cobalt tetraoxide nanorods Co with exposed crystal plane (110) 3 o 4 -110;
[0036] (b) Doping N atoms into cobalt tetraoxide nanorods Co 3 o 4 -110 surface, get tricobalt tetroxide catalyst N-Co 3 o 4 -110.
[0037] Specifically, step (a) includes:
[0038] (1) Mix ammonia water and polyol solution evenly, then add Na 2 CO 3 The solution is mixed evenly to obtain a mixed solution;
[0039] (2) Add Co(NO 3 ) 2 ·6H 2 The O solution is mixed evenly to obtain a sol; the mixed solution contains ammonia and Na 2 CO 3 Two alkaline solutions can be mixed with Co(NO 3 ) 2 ·6H 2 O reaction to synthesize Co(CO 3 ) 0.5 (OH) · 0.11 , which is calcined to eventually form Co 3 o 4 .
[0040] (3) Put the sol in a hydrothermal autoclave, and place the hydrothermal autoclave in a drying oven to heat up;
[0041] (4) washing and drying the product...
Embodiment 1
[0053] (a) Preparation of cobalt tetraoxide nanorods Co with exposed crystal plane (110) 3 o 4 -110
[0054] (1) Mix 10 mL ammonia water and 25 mL ethylene glycol solution evenly, then slowly add 1.5 mL, 1 mol L -1 Na 2 CO 3 The solution is mixed evenly to obtain a mixed solution;
[0055] (2) Add 5mL, 1 molL to the mixed solution -1 Co(NO 3 ) 2 ·6H 2 O solution, stirred continuously for 20 min to mix evenly to obtain a sol;
[0056] (3) Put the sol in a 100 mL hydrothermal autoclave, and gradually heat the hydrothermal autoclave to 170 o C and keep for 17h;
[0057] (4) Wash the product obtained in step (3) with deionized water at 60 o Dry at C for 12h;
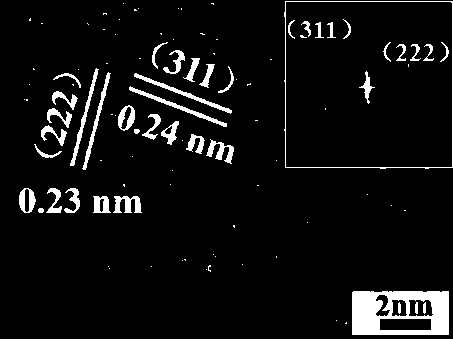
[0058] (5) Place the product obtained in step (4) in a muffle furnace for 300 o Calcined at C for 3h, the exposed crystal face of (110) cobalt tetraoxide nanorods Co 3 o 4 -110. After testing, it can be known that cobalt trioxide nanorods Co 3 o 4 The specific surface area of -110 is 36.8m 2 g -1 , the ...
Embodiment 2
[0061] (b) Doping N atoms in Co 3 o 4 -110 surface, get tricobalt tetroxide catalyst N-Co 3 o 4 -110
[0062] Get the cobalt trioxide nanorod Co in 0.2g embodiment 1 3 o 4-110 placed in the plasma cleaner, turn on the vacuum pump to vacuum for 5min, turn on the nitrogen switch and adjust the flowmeter to 50mLmin -1 Feed nitrogen into the instrument, set the power to 30W, and process for 20 minutes to obtain the tricobalt tetroxide catalyst N-Co 3 o 4 -110. After testing, it can be seen that the tricobalt tetroxide catalyst N-Co 3 o 4 The specific surface area of -110 is 52.3.8m 2 g -1 , the pore volume is 0.57ccg -1 .
[0063] image 3 For the prepared N-Co 3 o 4 SEM image of -110, image 3 N-Co is shown in 3 o 4 The nanorod morphology of -110 has a certain fracture, indicating that the N 2 The plasma has an etching effect on the cobalt tetroxide nanorods, so that it can expose a more active outer surface, so that the cobalt tetroxide catalyst produces mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com