Injection preparation of anti-CTLA-4 monoclonal antibody
A technology of CTLA-4 and monoclonal antibody, which is applied in the field of biomedicine, can solve the problems of patient death, reduce the efficacy of biological drugs, and instability, and achieve the effects of prolonging half-life, ensuring long-term stability, and avoiding denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] An injection preparation of an anti-CTLA-4 monoclonal antibody provided in Example 1 of the present invention, the preparation includes a pharmacodynamic molecule and a buffer solution, and the pharmacodynamic molecule is an anti-CTLA-4 monoclonal antibody with a protein content of 2-50 mg / mL,
[0049] The buffer solution contains the following components:
[0050]
[0051] The pH value of the buffer solution is 6.5-7.5.
[0052] Wherein: buffer salt includes one of phosphate buffer, Tris-hydrochloric acid buffer and disodium hydrogen phosphate-citric acid buffer; protein protection agent includes sucrose, mannitol, trehalose, arginine, glycine, proline The one in; the isotonic regulator is sodium chloride; the nonionic surfactant includes one in Tween 20, Tween 80, and poloxamer.
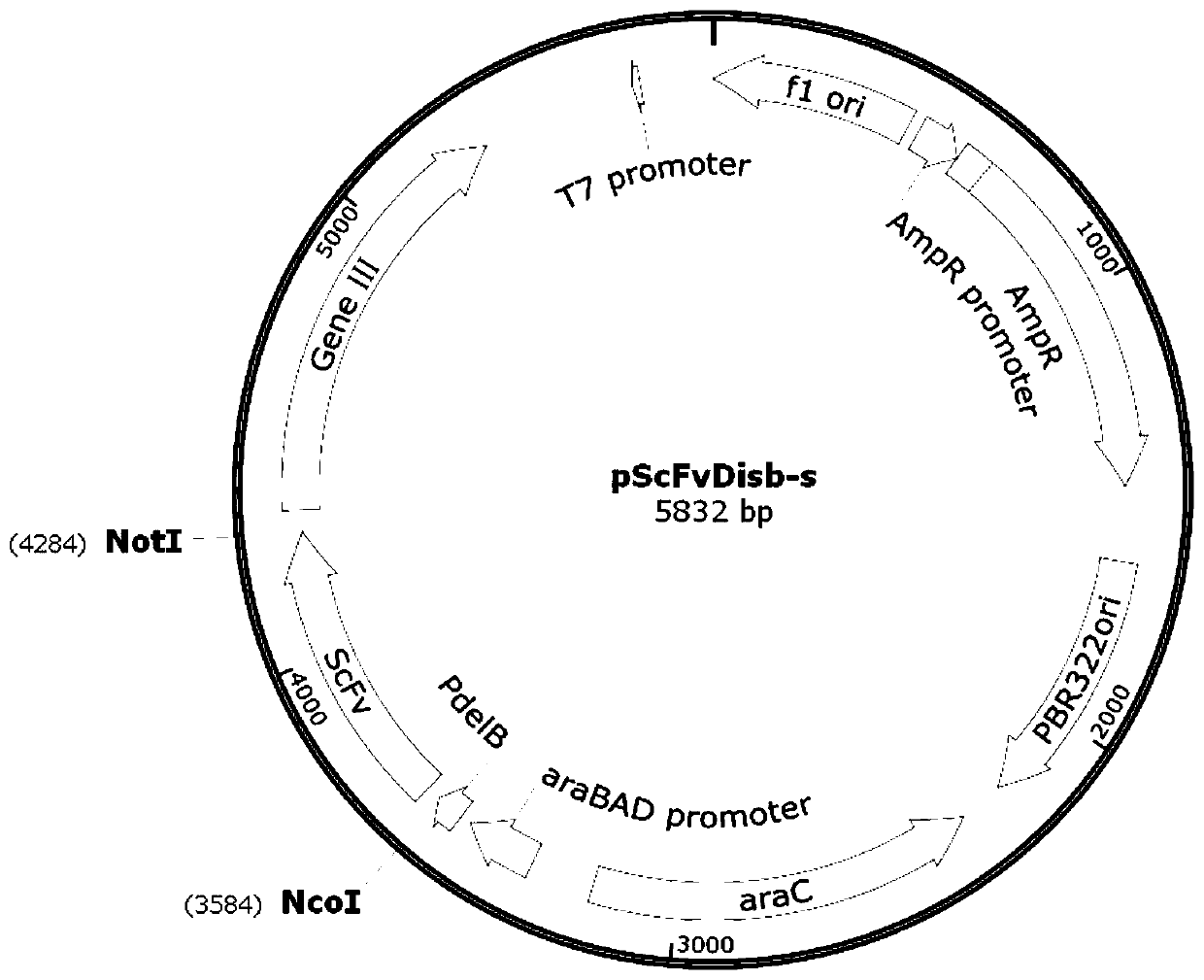
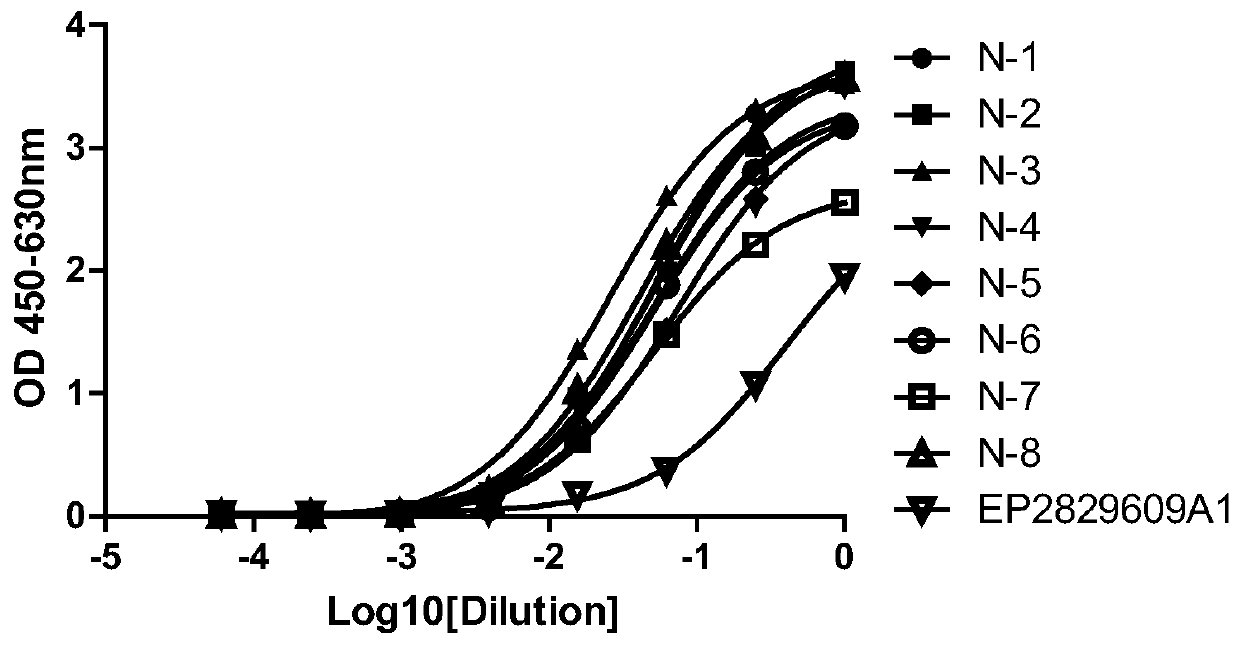
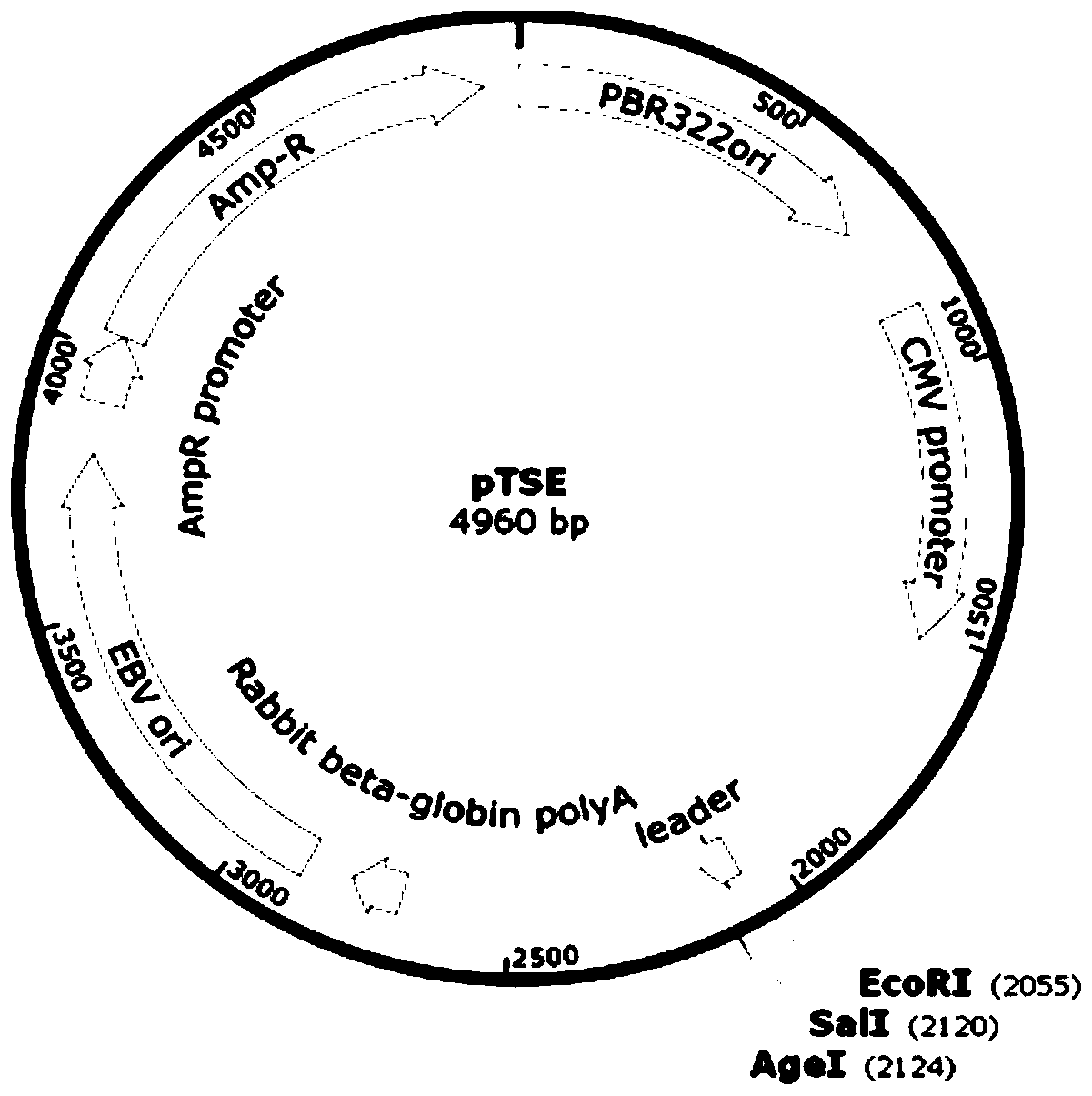
[0053] Among them, the anti-CTLA-4 monoclonal antibody is constructed by using a fully synthetic ScFv single-chain phage antibody library to obtain specific antibodies. The anti-CTLA-4 ...
Embodiment 2
[0090] Example 2 of the present invention provides an injection preparation of anti-CTLA-4 monoclonal antibody, which is based on Example 1 and further limits the protein content of the pharmacodynamic molecule to 10-20 mg / mL.
Embodiment 3
[0092] Embodiment 3 of the present invention provides an injection preparation of an anti-CTLA-4 monoclonal antibody, which further limits the content of buffer salt in the preparation to 10-20 mM on the basis of Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com