Catalyst for preparing citral through dehydrolinalool rearrangement reaction, preparation method of catalyst and method for preparing citral
A technology for dehydrolinalool and rearrangement reactions, applied in metal/metal oxide/metal hydroxide catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the constraints Application, high post-treatment requirements, poor stability of citral, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~20 and comparative example 1-2
[0097] The spherical carrier is prepared by a method well known to those skilled in the art, and the specific process is as follows: after the carrier (for example, silicon dioxide, aluminum oxide and titanium dioxide) is ground into powder, it is added to water, stirred evenly to form a carrier slurry, and then shaped Granulation to produce spherical supports (for example, spherical silica, spherical alumina, and spherical titania). Roast at 500-1100°C for 4-6 hours before use to obtain a particle size range of 3-5mm and a BET specific surface area range of 50-150m 2 / g spherical carrier.
[0098] Adopt impregnation method to prepare solid acid catalyst, specific process is as follows: heteropolyacid (for example, silicotungstic acid, phosphomolybdic acid or phosphotungstic acid) and optional auxiliary agent (for example, gold trichloride, rhenium trichloride, Indium trichloride or lanthanum trichloride heptahydrate) was dissolved in water to form an impregnating liquid, and...
Embodiment 23~ Embodiment 50 and comparative example 3-4
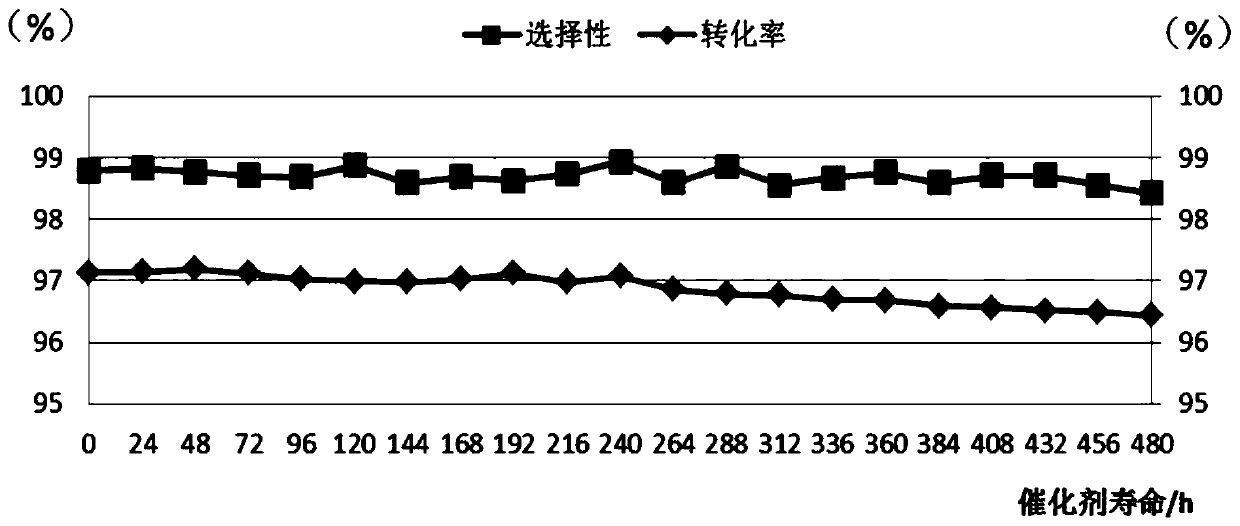
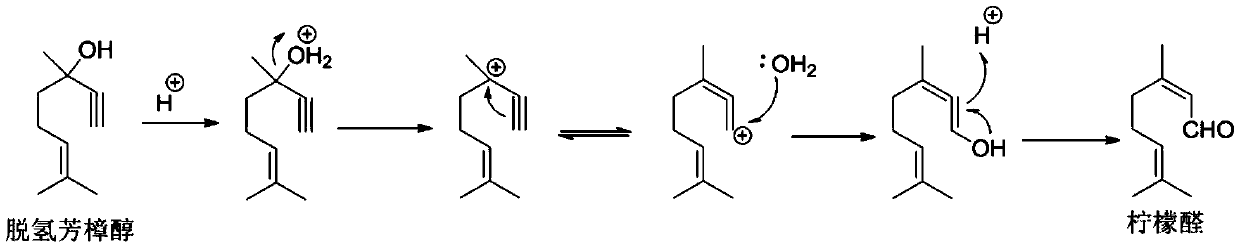
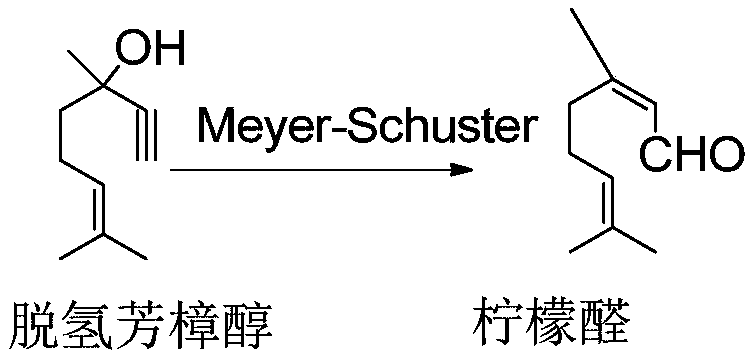
[0105] Select 24 solid acid catalysts prepared in Examples 1-22 and Comparative Examples 1-2 to screen, the method is: investigate the catalyst reaction performance with a fixed-bed reactor from top to bottom, and the fixed-bed reactor has an internal diameter of 20 mm and a length of 800mm stainless steel pipe. The catalyst loading was 5g and diluted to 20ml with glass beads. The catalysts prepared in Examples 1-22 and Comparative Examples 1-2 were loaded into a fixed-bed reactor, and activated under nitrogen protection for 2 hours at 400°C. Then cool to below 40°C and stop nitrogen purging. After mixing dehydrolinalool and solvent according to a certain mass ratio, the liquid phase passes through the catalyst bed, and under the condition of continuously feeding nitrogen, the fixed bed reactor is adjusted to the temperature required for the reaction, and the nitrogen pressure is adjusted to maintain a suitable Reaction conditions, rearrangement of dehydrolinalool to prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com