Method for preparing thermal stability vaccine based on calcium phosphate mineralization
A calcium phosphate mineralization and thermal stability technology, applied in the field of medicine and biotechnology, can solve the problem of low thermal stability of Streptococcus pneumoniae protein, stimulate humoral immunity and cellular immunity, improve thermal stability, improve subsidence effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
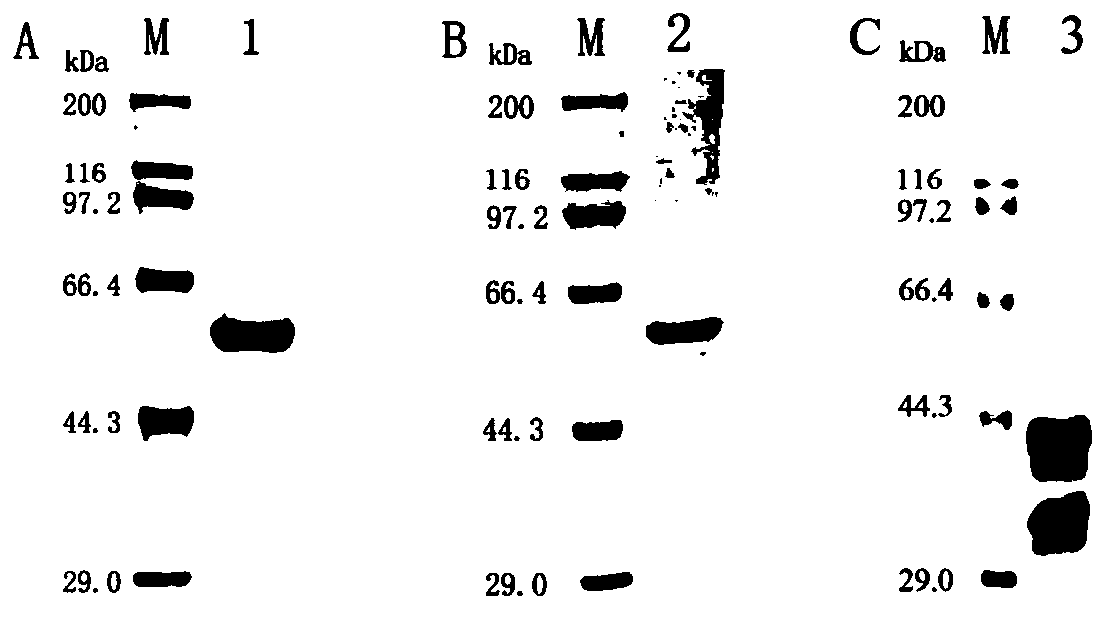
[0048] Expression, identification and purification of prokaryotic expression recombinant plasmids pET28a(+)-WT-PLY, pET28a(+)-ΔA146PLY and pET28a(+)-DnaJ in Escherichia coli
[0049] 1. The recombinant plasmids pET28a(+)-WT-PLY, pET28a(+)-ΔA146PLY and pET28a(+)-DnaJ were transformed into host bacteria BL21(DE3):
[0050] Add 10 μL of the recombinant plasmid to 200 μL of competent bacteria BL21(DE3), mix well, ice-water bath for 30 min; heat shock at 42°C for 90 s, ice-water bath for 2 min; add 800 μL LB medium, culture at 37°C×180rpm for 60min recovery; 3000rpm Centrifuge for ×5min, leave 200 μL of supernatant mixed with bacterial liquid, and spread it on LK plate; after incubation at 37°C for 13h, single clone colonies are picked.
[0051] 2. IPTG induces the massive expression of pET28a(+)-WT-PLY, pET28a(+)-ΔA146PLY and pET28a(+)-DnaJ:
[0052] E.coli BL21-WT-PLY and E.coli BL21-ΔA146PLY strains were activated in 5 mL of LB liquid medium containing 50 ng / mL kanamycin (kana)...
Embodiment 2
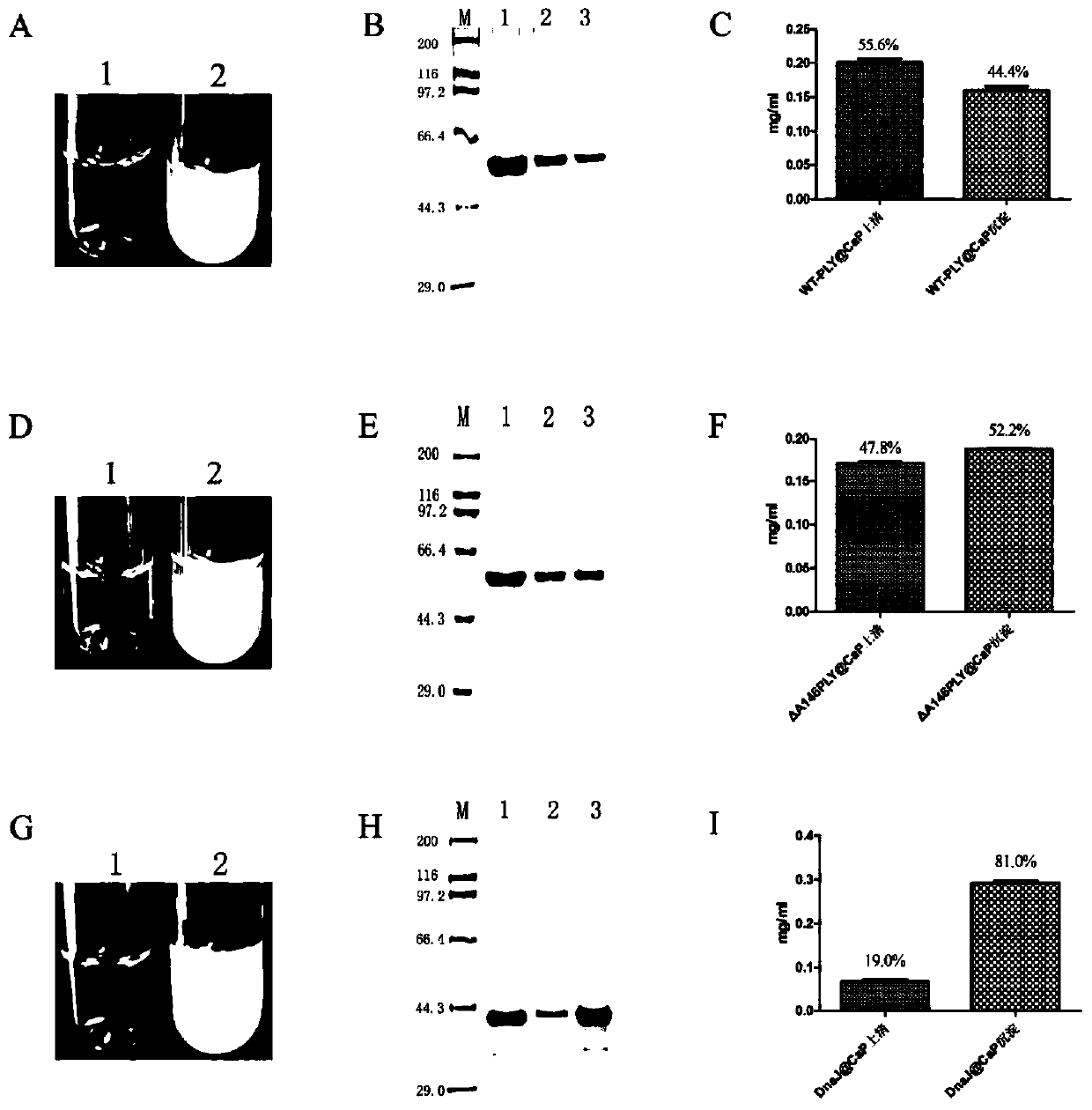
[0063] In situ calcium phosphate mineralization and biological characteristics analysis of recombinant proteins WT-PLY, ΔA146PLY and DnaJ
[0064] 1. In situ calcium phosphate mineralization of recombinant proteins WT-PLY, ΔA146PLY and DnaJ
[0065] 360 μg proteins WT-PLY, ΔA146PLY and DnaJ were mixed with 8.8 μmol CaCl, respectively. 2 , 8.8 μmol MgCl 2 and 5.28 μmol Na 2 HPO 4 / NaH 2 PO 4 (pH=7.5) buffers were mixed together and the remaining volume was ddH 2 O (pH=7.5) was made up to 1 mL. The mineralization system and the magnetic beads treated with DEPC were placed in a beaker treated with DEPC, and stirred at 4 °C for 4 h under the action of a magnetic stirrer before use. Take 40 μL of the mineralized suspension respectively, centrifuge at 4°C*3000rpm*5min, separate the supernatant and the precipitate, add 10 μL of 5× loading buffer to 40 μL of supernatant, add 50 μL of 1× loading buffer to the precipitate, boil in boiling water for 10 min, and take 20 μL of sampl...
Embodiment 3
[0080] Analysis of proteinase K degradation resistance of recombinant proteins WT-PLY, ΔA146PLY and DnaJ and their mineralized nanoparticles
[0081] Take 25 μL of WT-PLY protein and mineralized WT-PLY@CaP suspension, and treat them with 15 μL of 40 μg / mL proteinase K for 0, 1, 5, 10, and 15 min respectively; take 25 μL of ΔA146PLY protein and ΔA146PLY@CaP with 15 μg / mL 15 μL of proteinase K was treated for 0, 1, 5, 10, and 15 min, respectively; 25 μL of DnaJ protein and DnaJ@CaP were treated with 15 μL of proteinase K at 10 μg / mL, respectively, for 0, 1, 5, 10, and 15 min. Add 1 μL PMSF to stop the enzymatic hydrolysis reaction, add 10 μL 5×loading buffer, boil in boiling water for 10 min, take 20 μL samples into the sample wells of SDS-PAGE gel, run the gel at 80 V for 40 min, run the gel at 120 V for 80 min; use Coomassie brilliant blue Dyeing with dye solution for 2min (heating on high heat in microwave oven for 2min), use ddH 2 O microwave heating for 10 min (repeated tw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com