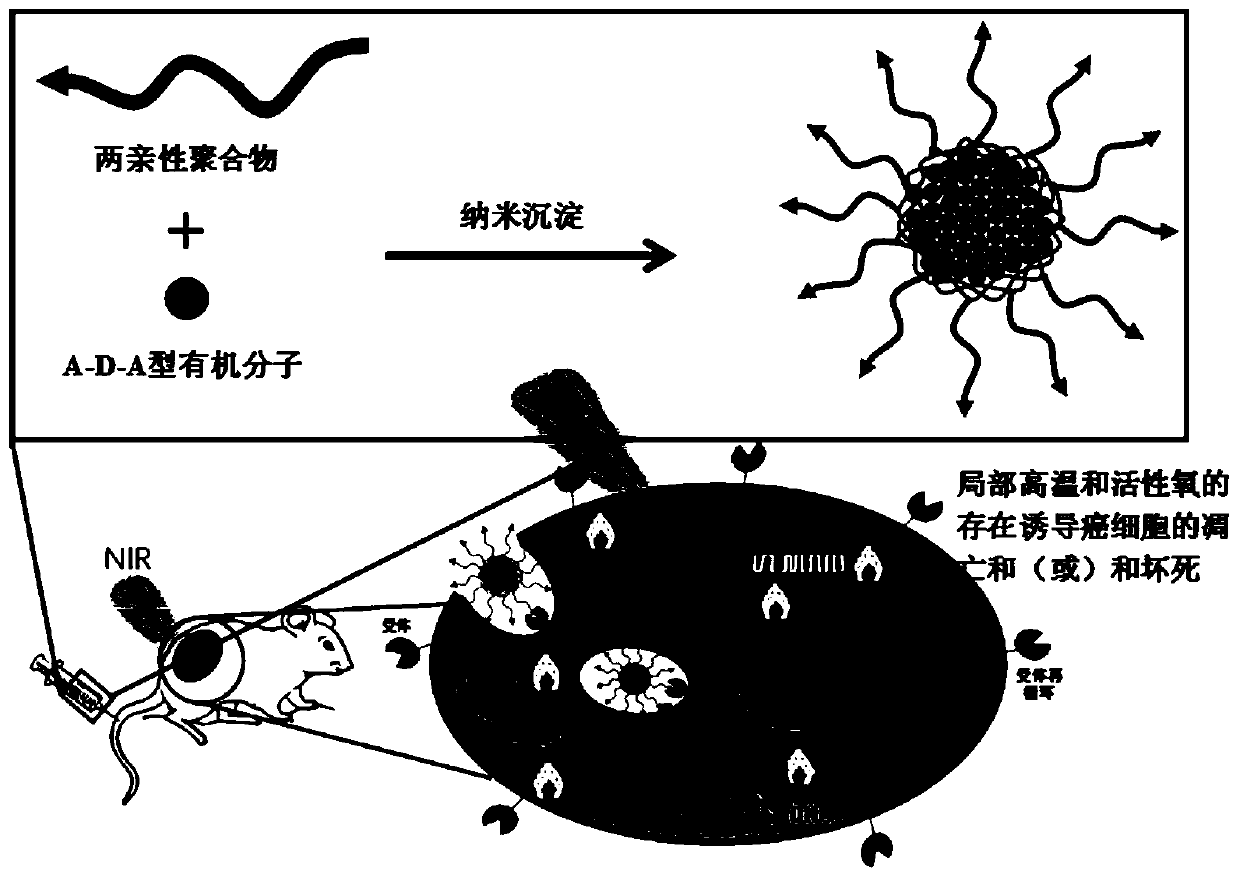
Preparation method and application A-D-A type organic molecule/amphiphilic polymer composite nanoparticles
A composite nanoparticle, A-D-A technology, applied in non-active ingredients medical preparations, active ingredients-containing medical preparations, general/multifunctional contrast agents, etc., to achieve good photochemical properties, good application prospects, good tumor selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
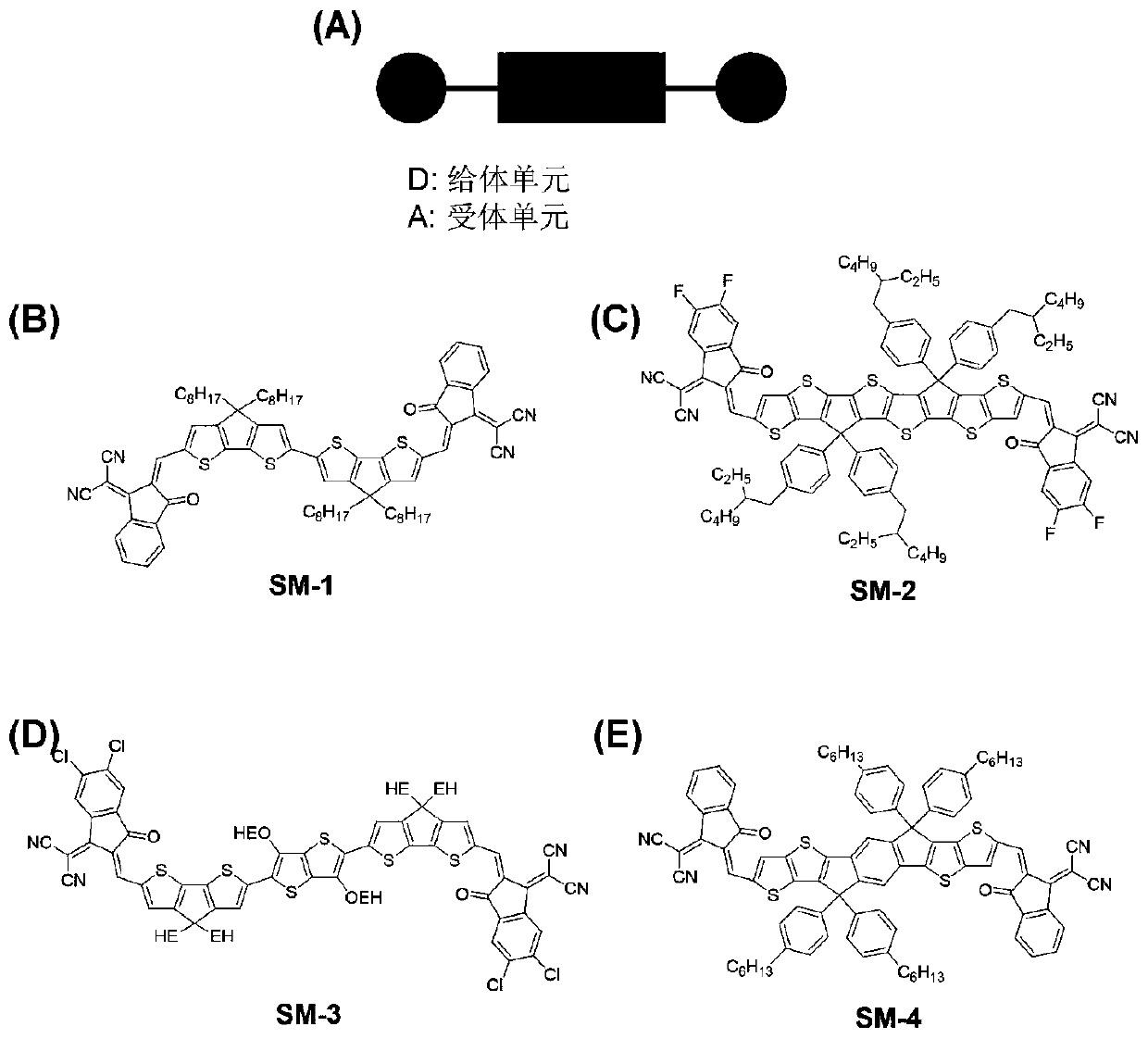
[0044] 1) Synthesis of SM-1
[0045] The schematic diagram of the synthesis of SM-1 is shown in Figure 12 As shown, first add (1)(6-bromo-4,4-bis(2-ethylhexyl)-4H-cyclopentane[2,1-b:3,4-b ']dithiobenzene-2-carbal) (0.2g, 0.392mmol), bis(pinacolate)diboron (0.12g, 0.47mmol), potassium acetate (1.154g, 11.759mmol) and DMSO (4mL) , after purging with nitrogen for 0.5 hours and adding 1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (9.6mg, 1.2×10 - 2 mmol). The mixture was heated to 80° C. and stirred for 8 h. After the reaction, it was naturally cooled to room temperature and washed with water. The aqueous layer was extracted with chloroform, treated with anhydrous sodium sulfate, and then concentrated. The obtained primary product was purified by column chromatography (eluent PE:DCM=6:1, v / v) to obtain 0.17 g (85%) of product (2) as a dark red solid.
[0046] Intermediate (2) (0.3 g, 0.349 mmol) and indenophenone dicyano (3) (0.407 g, 2.1 mmol) were dissolved in ...
Embodiment 2
[0054] 1) Synthesis of SM-1
[0055] The synthesis of SM-1 is shown in Example 1.
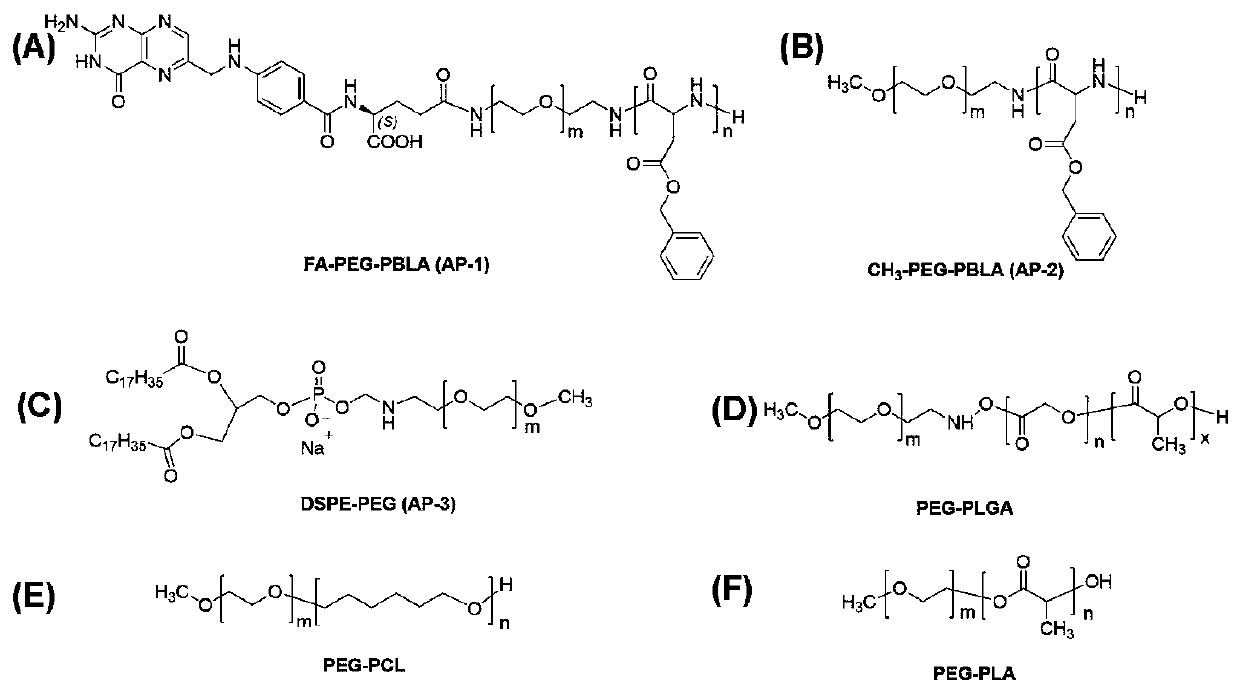
[0056] 2) Synthesis of amphiphilic high son AP-2
[0057] Add 150mg BLA-NCA and 0.3mL DMF to a round bottom flask, add 3mL CH 3 -PEG-NH 2 Chloroform solution (303mg), the reaction was carried out at 40°C for 48 hours. After the reaction, the product was precipitated with excess glacial ether and centrifuged.
[0058] 3) Preparation of NPs-2 nanoparticles
[0059] The present invention prepares NPs-2 by nanoprecipitation method, first dissolves 5mgSM-1 in 1mLCHCl 3 , and then the SM-1 solution was slowly and uniformly added dropwise to 50mLAP-2 in DMSO solution (0.5mg / mL). CHCl was then removed under nitrogen flow 3 , to be CHCl 3 After complete removal, the solution was transferred to a dialysis bag (molecular weight cut-off: M W 3.5 kDa) to remove DMSO followed by lyophilization.
Embodiment 3
[0061] 1) Synthesis of SM-1
[0062] The synthesis of SM-1 is shown in Example 1.
[0063] 2) Preparation of NPs-3 nanoparticles
[0064] The present invention adopts the method of nanoprecipitation to prepare NPs-3, at first 5mgSM-1 is dissolved in 1mLCHCl 3 Then, the SM-1 solution was slowly and evenly added dropwise to 50 mL of a DMSO solution of distearoylphosphatidylethanolamine-polyethylene glycol (AP-3) (0.5 mg / mL). CHCl was then removed under nitrogen flow 3 , to CHCl 3 After complete removal, the solution was transferred to a dialysis bag (molecular weight cut-off: M W 3.5 kDa) to remove DMSO followed by lyophilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com