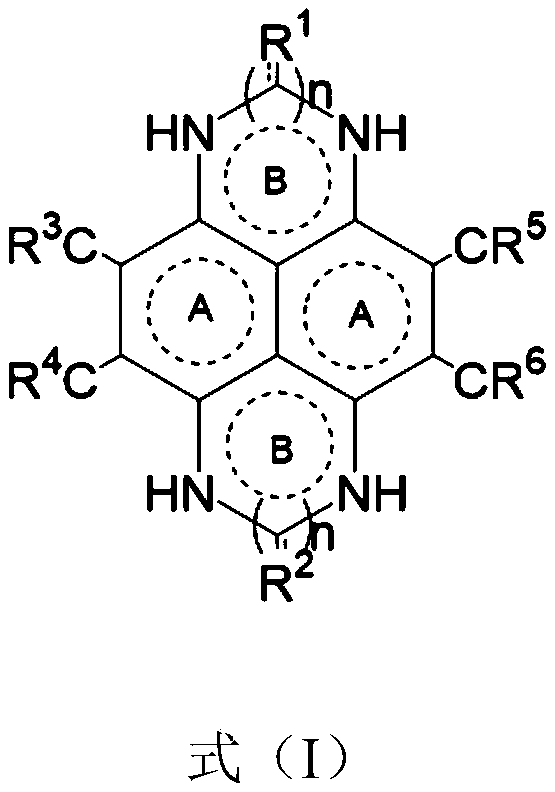
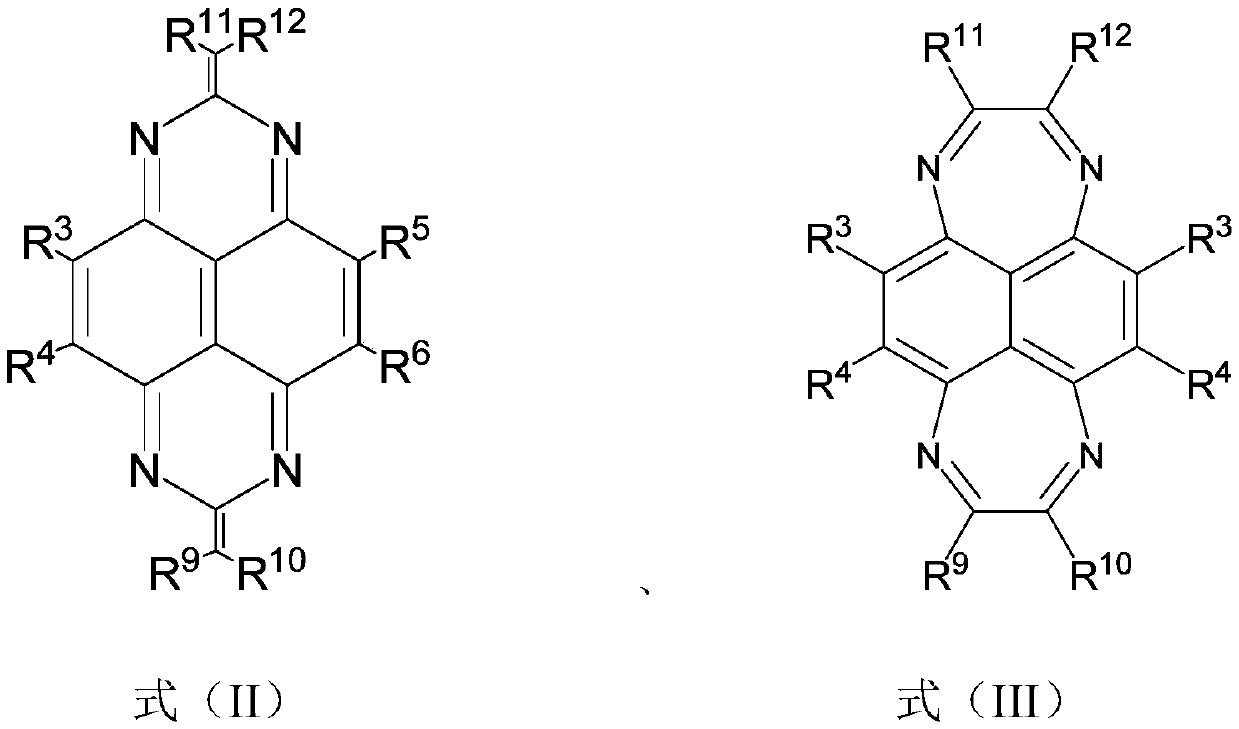
Fused ring aryl compound, electronic device and application thereof
A technology for condensed ring aromatic groups and electronic devices, applied in the field of optoelectronic materials, can solve the problems of short lifespan of P-doped materials, and achieve the effects of reducing annihilation, enhancing electron injection and transport capabilities, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] Synthesis of intermediate 1-P-1: In a 50 ml three-necked flask, 2,3,6,7-tetrabromonaphthalene-1,4,5,8-tetraketone (5.03 g, 0.01 mol ), N-methylpyrrolidone (NMP, 20 ml), sodium carbonate (0.01mol), K 4 [Fe(CN) 6 ](2.5mmol),Pd(OAc) 2 (1mmol), heated to 120 degrees Celsius, stirred for 20 hours, after the reaction was completed, cooled to room temperature, filtered, removed the solvent, and the crude product was passed through a chromatographic column (n-hexane: ethyl acetate: dichloromethane=10:2:2) , to obtain intermediate 1-P-1 (1.8 g, yield 63%).
[0041] Synthesis of P-1:
[0042] The first step: in a 50 ml three-necked flask, add 1-P-1 (2.9 grams, 1 equivalent, 0.01mol), urea (2.4 grams, 4 equivalents), and ethanol (10 milliliters) to reflux for 10 hours under a nitrogen atmosphere. After the reaction was completed, cooled to room temperature, quenched by adding 10 ml of water, extracted with ethyl acetate (20 ml*3), dried over anhydrous sodium sulfat...
Embodiment 2
[0046]
[0047] Synthesis of P-4: In a 50 ml three-necked flask, add 1,4,5,8-tetrafluoro-2,3,6,7-tetracyanonaphthalene (3.0 g, 0.01 mol) under nitrogen protection, di Amino maleic dicyanide (2.16 g, 0.02 mol), DMF (10 ml), sodium tert-butoxide (1 mmol), heated to 120 ° C, stirred for 5 hours, after the reaction was completed, quenched with water, extracted with dichloromethane , the organic layer was dried with anhydrous magnesium sulfate, the solvent was removed, and the product was beaten three times with (n-hexane: dichloromethane: acetonitrile=10:2:3), and once with acetonitrile to obtain P-4 (1.51 grams, yield 35%) .
[0048] Elemental Analysis: C 22 N 12 Theoretical: C, 61.12; N, 38.88; Found: C, 61.16; N, 38.84; HRMS (ESI) m / z(M): Theoretical: 432.0369; Found: 432.0361.
Embodiment 3
[0050] Fabrication of organic electroluminescent devices:
[0051] The ITO transparent substrate is placed in the evaporation equipment, wherein ITO (indium tin oxide) is used as the anode layer 1, and the 10nm hole injection layer 2, the 100nm hole transport layer 3 (HTL), and the 50nm organic light-emitting layer 4 ( EML), 40nm electron transport layer 5 (ETL), 1nm electron injection layer 6 (EIL), 80nm cathode layer 7, where N,N'-diphenyl-N,N'-(1-naphthyl)- 1,1'-biphenyl-4,4'-diamine as hole transport layer 3, 3 wt% Ir(piq) 3 : CBP is used as the organic light-emitting layer 4, BPhen is used as the electron transport layer 5, LiF is used as the electron injection layer 6, and Al is used as the cathode layer 7 so that the device is formed as figure 1 The specific structure shown.
[0052] step:
[0053] (1) Substrate cleaning: the transparent motor substrate coated with ITO is ultrasonically treated in an aqueous cleaning agent (composition and concentration of the aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com