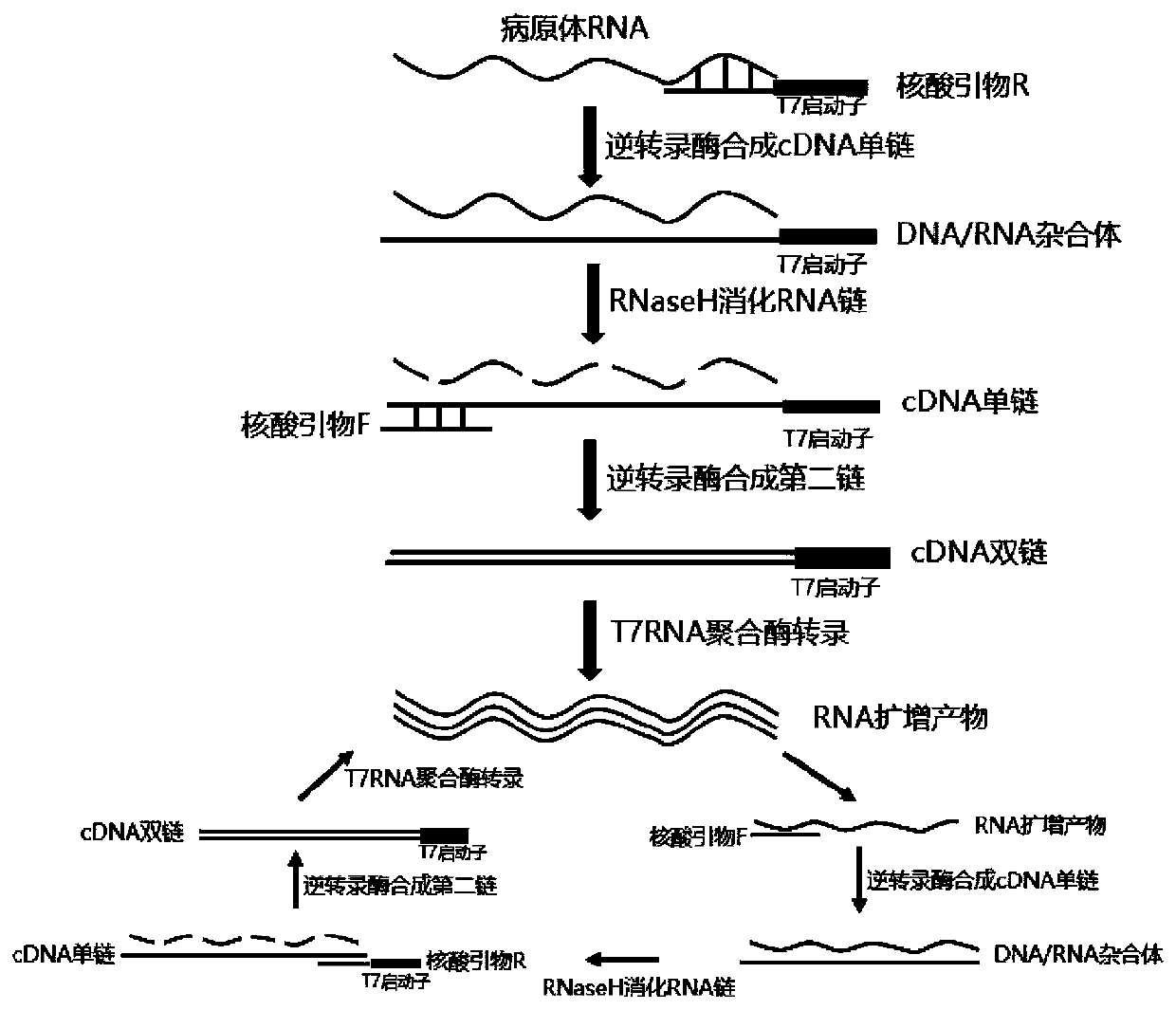
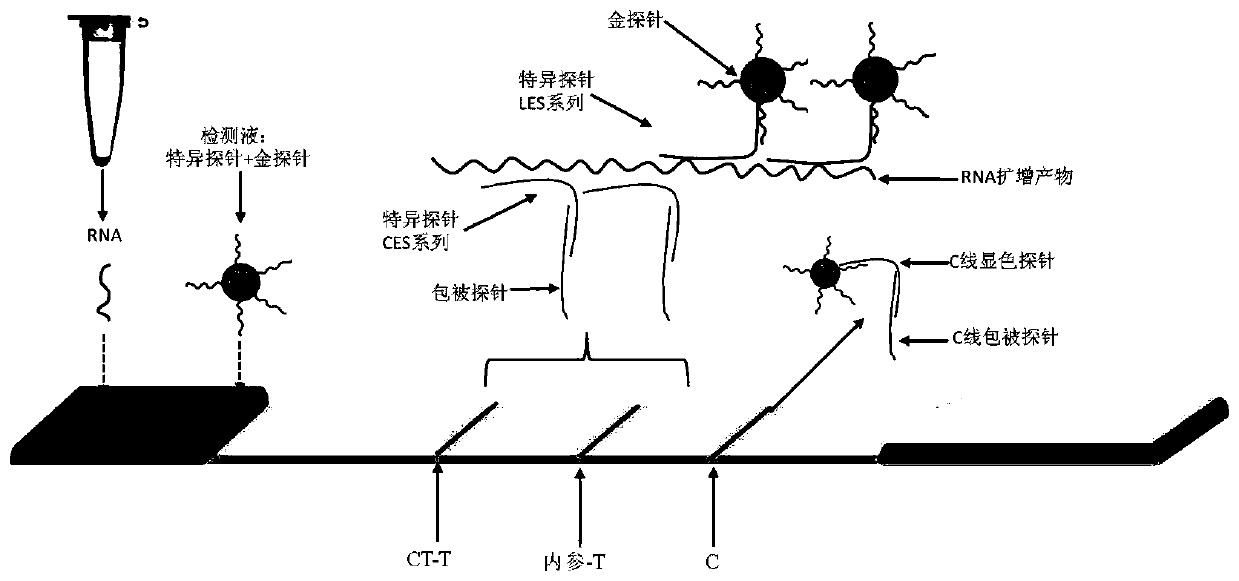
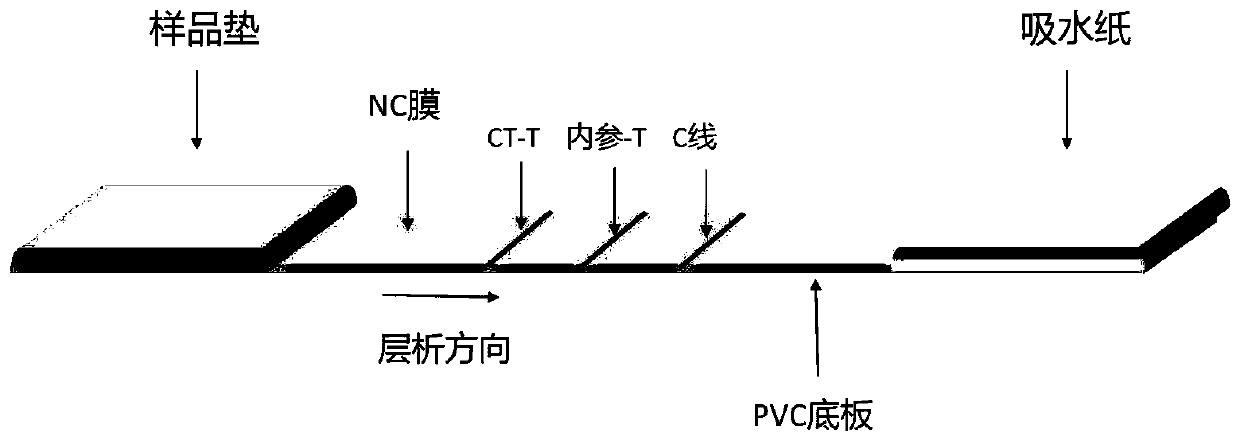
Chlamydia trachomatis detection colloidal gold chromatography kit and application thereof
A technology for Chlamydia trachomatis and a kit, which is applied to the determination/inspection of microorganisms, biochemical equipment and methods, microorganisms, etc., can solve problems such as being unsuitable for processing a large number of samples, time-consuming, and low sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] [Example 1] Preparation of nucleic acid detection test strips
[0086] The main raw materials required for the preparation of nucleic acid detection test strips: nitrocellulose membrane (NC membrane), sample pad, absorbent paper, PVC bottom plate, etc.
[0087] 1. Spray film:
[0088] Detection line CT-T: Capable of capturing and binding CT-specific probe CES sequence, 10μM CT-coated probe, spray film volume: 2-3μL / cm;
[0089] Detection line internal reference-T: capable of capturing and binding internal reference-specific probe CES sequence, 10 μM internal reference coated probe, spray film volume: 2-3 μL / cm;
[0090] Quality control line C-line: capable of capturing and combining C-line chromogenic probe sequence, 10μM C-line coated probe, spray film volume: 2-3μL / cm;
[0091] After spraying the film, it will be automatically cross-linked once in the ultraviolet cross-linking instrument, put it in a clean incubator at 37°C for 2 hours, and store it in a dry environme...
Embodiment 2
[0094] [Example 2] Sensitivity Test
[0095] The minimum detection limit of Chlamydia trachomatis derived from ATCC VR-346 is serially diluted, and the dilution of each gradient is repeated 3 to 5 times, and each copy is repeated 20 times, and 90% to 95% of positive detections will be obtained. The level of the rate is used as the minimum detection limit, and the detection results are as follows:
[0096] CT minimum detection limit detection
[0097] Table 1.1 Detection experimental data of CT with different titers
[0098]
[0099] Table 1.2 Experimental data of the lowest detection limit of CT
[0100]
[0101]
[0102] In summary, the sensitivity of the kit of the present invention for detecting CT is 5.0 IFU / mL.
Embodiment 3
[0103] [Example 3] Specificity Verification
[0104] 1. Test Strains
[0105] After extracting nucleic acids from different microorganisms, they are tested to verify the specificity of the design of the primers and probes of the kit of the present invention. Relevant pathogens and titer information are as follows:
[0106] Table 2 Specificity verification test strain information
[0107]
[0108] 2. Specificity Test
[0109] After extracting nucleic acids from different microorganisms, they are tested to verify the specificity of the design of the primers and probes of the kit of the present invention. Relevant pathogens and titers are as follows:
[0110] Table 3 Specificity verification test results
[0111]
[0112]
[0113] 3. Conclusion
[0114] As can be seen from the above data, the detection results of the kit of the present invention to these microorganisms are all negative, proving that there is no cross-reaction between the kit of the present inventio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com