Arylamine derivative and organic light-emitting device thereof
A technology of organic light-emitting devices and derivatives, which is applied in the direction of light-emitting materials, organic chemistry, and electric solid-state devices. Good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0122] The preparation and formation methods of each layer in the organic light-emitting device are not particularly limited, and vacuum evaporation method, spin coating method, vapor deposition method, blade coating method, laser thermal transfer method, electrospray coating method, and slit coating method can be used. Any one of the method and the dip coating method, the method of vacuum evaporation is preferably used in the present invention.
[0123] The organic light-emitting device of the present invention can be widely used in the fields of panel display, lighting source, flexible OLED, electronic paper, organic solar cell, organic photoreceptor or organic thin film transistor, signboard, signal lamp and the like.
Synthetic example 1
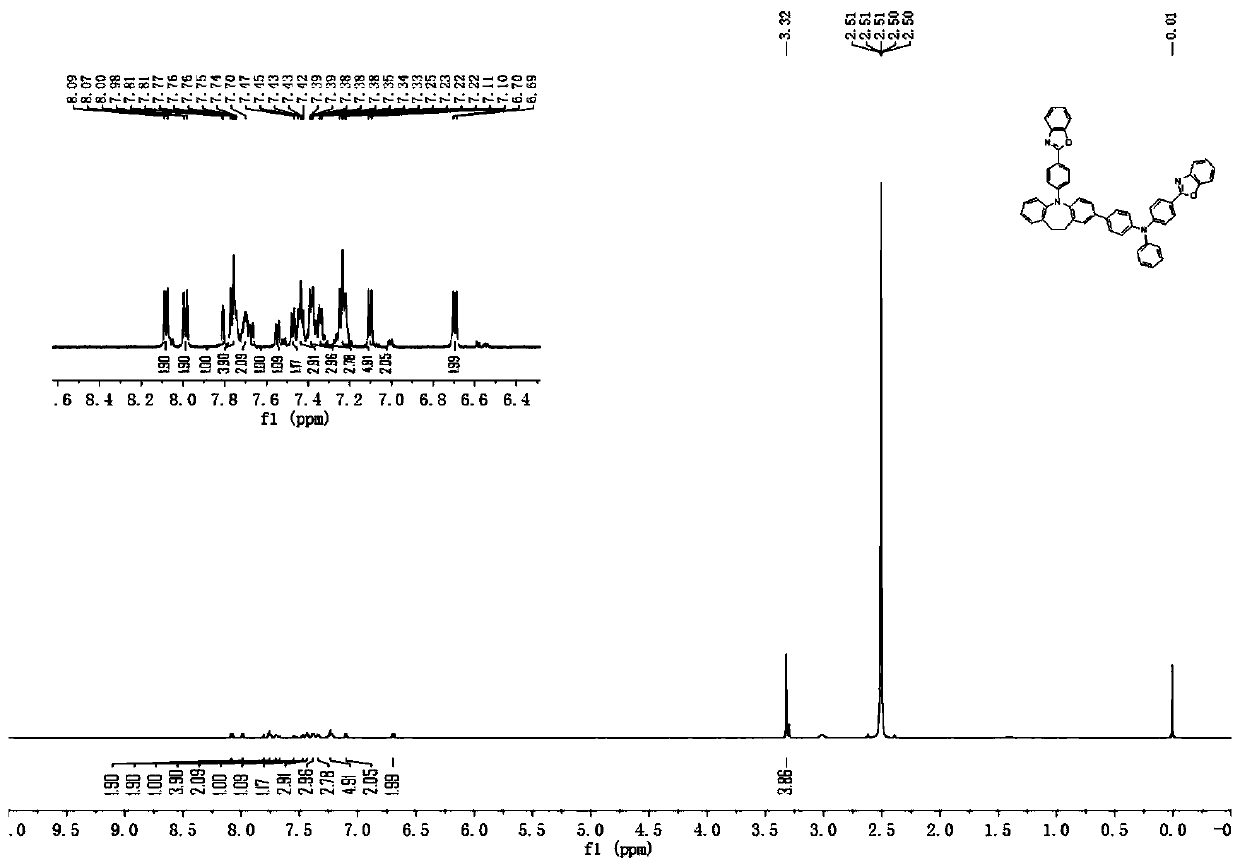
[0131] [Synthesis Example 1] Synthesis of Compound 1
[0132]
[0133] Synthesis of Intermediate 1-1
[0134] Under nitrogen protection, weigh 3-chloroiminodibenzyl (18.83g, 0.082mol), compound 2-(4-bromophenyl) benzoxazole (24.08g, 0.083mol), cuprous iodide (7.8 g, 0.041mol), ethylenediamine (2.8mL, 0.041mol) and cesium carbonate (80g, 0.246mol), and were added to toluene (250mL) in the above order, and stirred at reflux. Extraction with ethyl acetate, distillation under reduced pressure, dichloromethane and hexane to get intermediate 1-1 (28.78g, 83%). HPLC detection solid purity ≧99.3%.
[0135] Synthesis of Intermediate 1-2
[0136] Under nitrogen protection, toluene (600mL), 4-(2-benzoxazolyl)aniline (44.15g, 0.21mol), 1-bromobenzene (32.97g, 0.21mol), acetic acid Palladium (0.61 g, 0.0027 mol), sodium tert-butoxide (33.7 g, 0.351 mol) and tri-tert-butylphosphine (10.8 mL of a 1.0 M solution in toluene, 0.0108 mol). And react under the condition of reflux for 2 ho...
Synthetic example 2
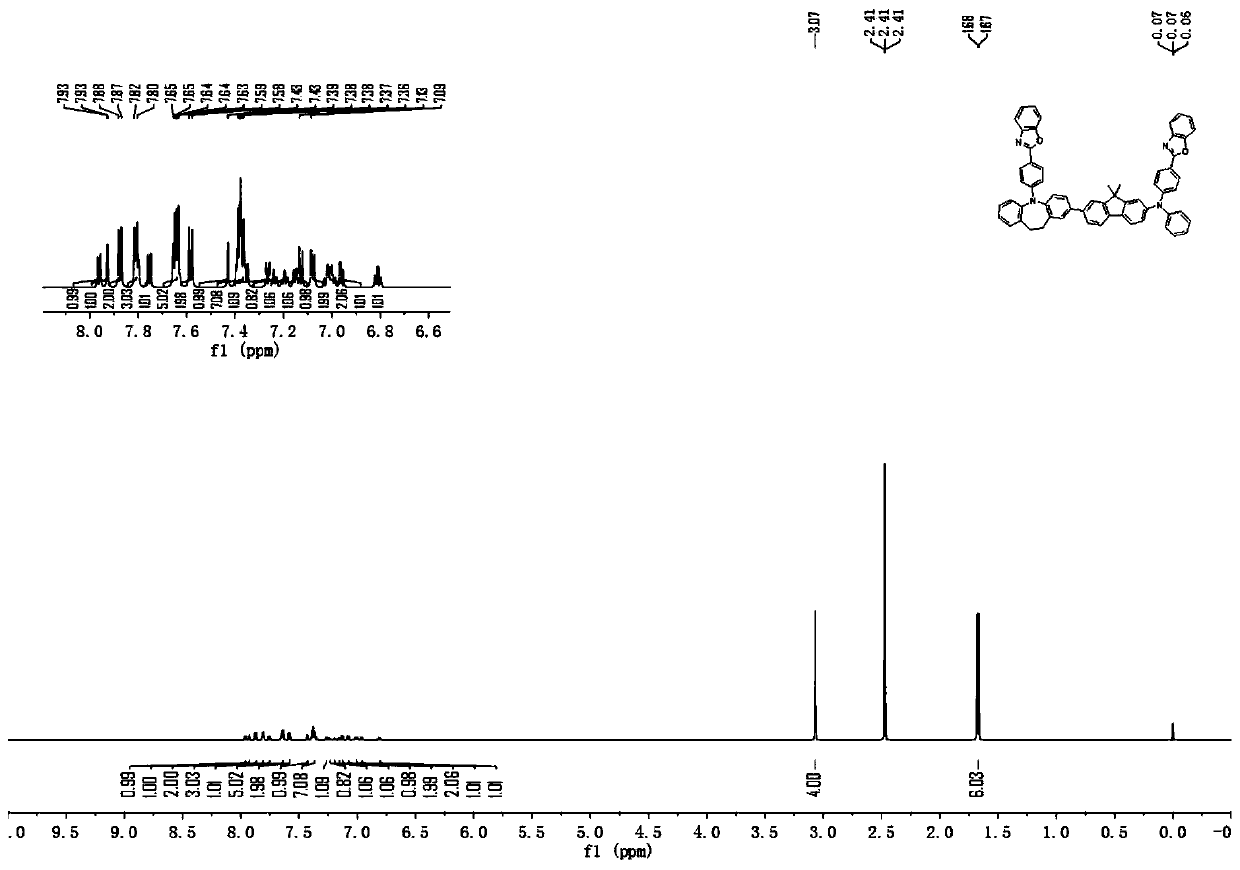
[0142] [Synthesis Example 2] Synthesis of Compound 12
[0143]
[0144] The 4-bromophenylboronic acid in Synthesis Example 1 was replaced by equimolar e-12, and the other steps were the same to obtain compound 12 (36.33 g, yield about 84%), and the purity of the solid was detected by HPLC ≧99.0%.
[0145] Mass Spectrum m / z: 864.39 (calculated: 864.35). Theoretical element content (%)C 61 h 44 N 4 o 2 : C, 84.70; H, 5.13; N, 6.48; O, 3.70 The measured element content (%): C, 84.70; H, 5.14; N, 6.47; O, 3.70. 1 H NMR (600MHz, DMSO) (δ, ppm): 7.96(d,1H), 7.93(d,1H), 7.89–7.85(m,2H), 7.83–7.78(m,3H), 7.75(d,1H ),7.66–7.62(m,5H),7.60–7.56(m,2H),7.43(d,1H),7.40–7.33(m,7H),7.26(dd,1H),7.25–7.22(m,1H ),7.20(ddd,1H),7.15(dd,1H),7.13(d,1H),7.08(dd,2H),7.03-6.98(m,2H),6.96(dd,1H),6.81(td, 1H), 3.07(s, 4H), 1.67(d, 6H). The above results confirmed that the obtained product was the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com