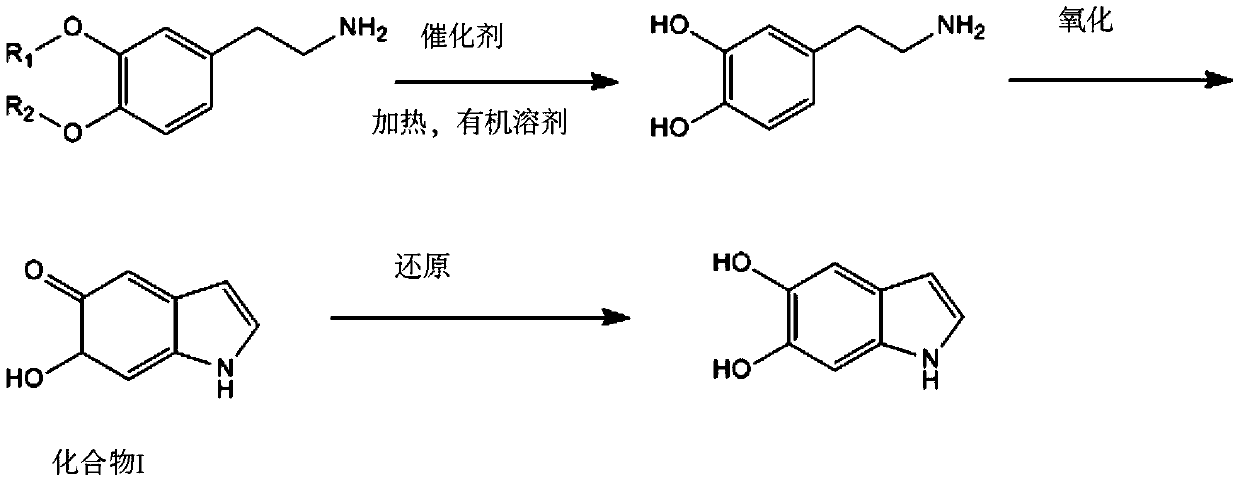
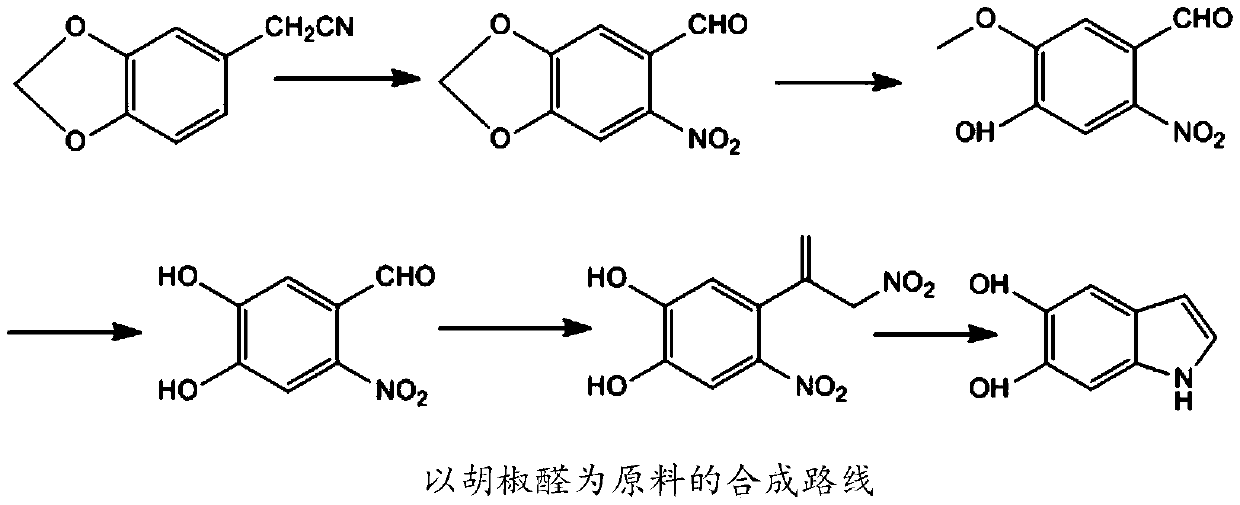
A method for efficiently preparing 5,6-dihydroxyindole
A technology of dihydroxyindole and dialkoxyphenethylamine, which is applied in the field of efficient preparation of the compound 5,6-dihydroxyindole, which can solve cumbersome reaction steps, long experiment time, and unsuitability for large-scale production of processes, etc. problem, to achieve the effect of simple post-reaction treatment, short reaction process, and easy long-term storage and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of efficient preparation method of 5,6-dihydroxyindole, comprises the steps:
[0033] (1) At room temperature, weigh 3.6320g of 3,4-dimethoxyphenethylamine (about 0.02mol, molecular weight 181.23), place it in a dry and clean three-neck round bottom flask, dissolve it in 50mL of glacial acetic acid, and stir evenly Make 3,4-dimethoxyphenethylamine completely dissolved;
[0034] (2) Slowly add 20 mL of 37% hydrochloric acid solution (about 0.24 mol) to the reaction system, gradually heat up to 160° C., and reflux at 800 r / min for 6 hours under nitrogen protection;
[0035] (3) After the reaction is over, concentrate under reduced pressure to remove excess acid, add deionized water to the reaction solution, and clean the residual product on the wall. The dosage is 10 mL, repeat three times until there is no obvious residue on the wall, and then extract with 30 mL of chloroform Three times, the water layer was taken, the solvent was removed by rotary evaporation, a...
Embodiment 2
[0041] A kind of efficient preparation method of 5,6-dihydroxyindole, comprises the steps:
[0042] (1) At room temperature, weigh 7.2320g (about 0.04mol, molecular weight 181.23) of 3,4-dimethoxyphenethylamine, place it in a dry and clean three-neck round bottom flask, dissolve it in 100mL of glacial acetic acid, and stir evenly Make 3,4-dimethoxyphenethylamine completely dissolved;
[0043] (2) Slowly add 50 mL of 48% hydrobromic acid solution (about 0.44 mol) to the reaction system, gradually heat up to 160° C., and reflux at 800 r / min for 8 hours under nitrogen protection;
[0044] (3) After the reaction is over, concentrate under reduced pressure to remove excess acid, add deionized water to the reaction solution, and clean the residual product on the wall. Methyl chloride was extracted 3 times, the water layer was taken and the solvent was removed by rotary evaporation, and dried in a vacuum oven for 1 hour to obtain 7.3256 g of white solid powder dopamine hydrobromide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com