Method for preparing pantoprazole nitrogen oxide
A technology for nitrogen oxides and pantoprazole, which is applied in the field of preparation of pantoprazole nitrogen oxides, can solve the problems of increasing manpower, resource costs, input of large manpower and material resources, and inability to guarantee the time limit, and achieves the preparation of The method is simple and easy to operate, saves production costs, and improves the effect of cheapness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
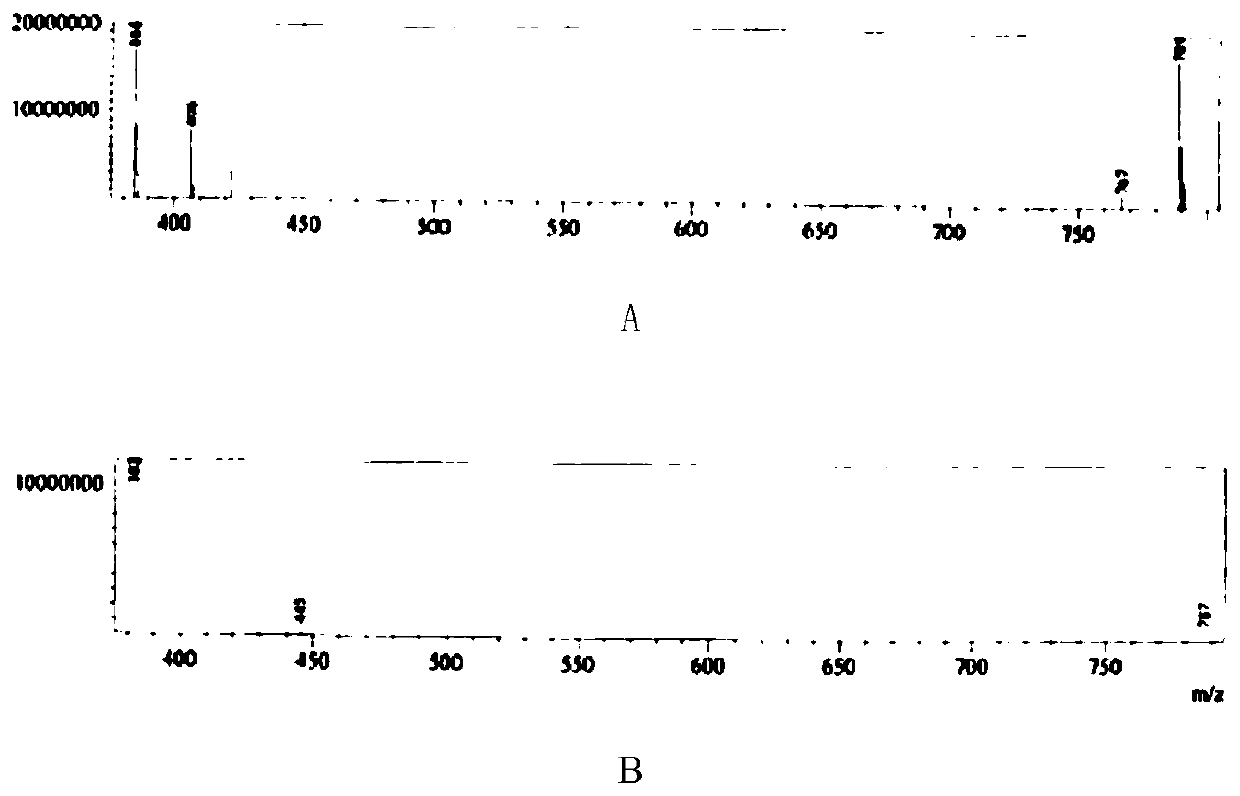
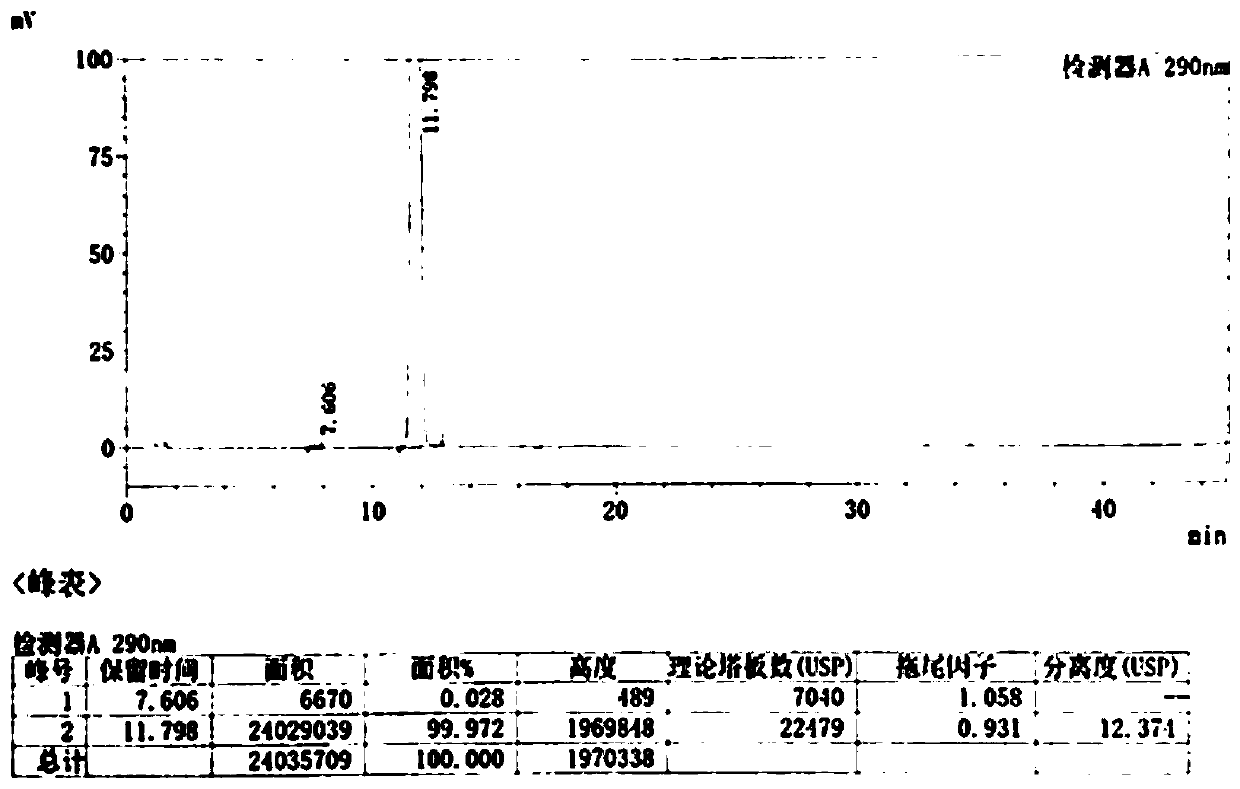
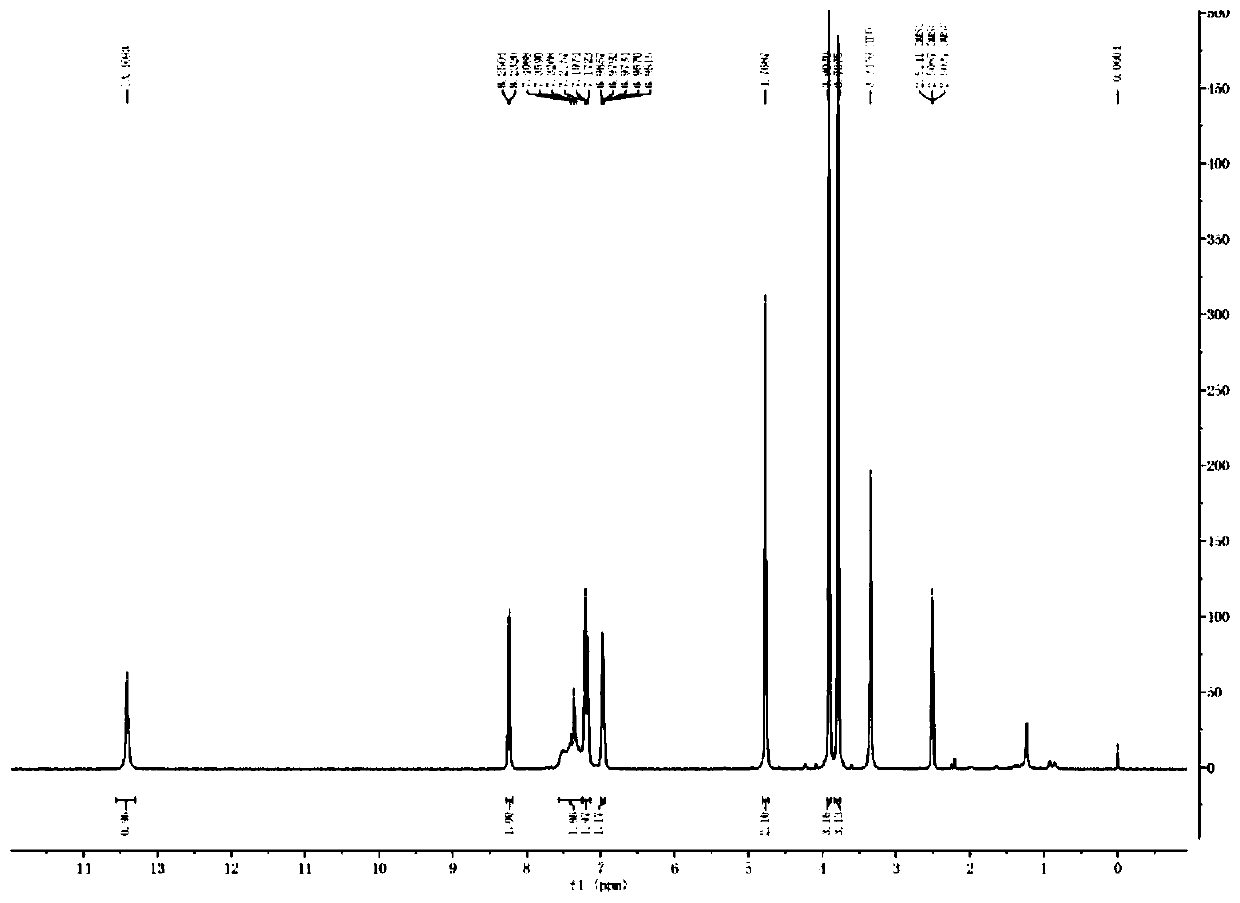
Image
Examples
Embodiment 1
[0039] (1) Add 20% sodium hydroxide compound (10ml, 5mmol) to a 25ml reaction flask, add 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (1g, 4.4mmol), and stir for 30 minutes , standing for stratification, discarding the upper aqueous phase, and retaining the lower layer of light yellow oil;
[0040] (2) Dissolve the oily matter in step (1) with dichloromethane (20ml), cool down to 2°C, add m-chloroperoxybenzoic acid (1.5g, 7.4mmol) in batches, after addition, 0-10°C React for 2 hours. After the reaction was completed, the reaction solution was washed with sodium metabisulfite solution, and the layers were separated. Dichloromethane was distilled off under reduced pressure to obtain a brown oil;
[0041](3) Add methanol (20ml) to the brown oil in step (2), stir to dissolve, add 5-difluoromethoxy-2-mercapto-1H-benzimidazole, cool to 0-10°C, add 20% aqueous sodium hydroxide solution (22ml) was added and reacted at 0-10°C for 2 hours. After the reaction, adjust the pH to ...
Embodiment 2
[0044] (1) Add 10% potassium carbonate aqueous solution (10ml) to the 25ml reaction bottle, add 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (0.5g, 2.2mmol), stir for 30 minutes, static Separate the layers, discard the upper aqueous phase, and keep the lower layer of light yellow oil;
[0045] (2) Dissolve the oil in step (1) with tetrahydrofuran (15ml), cool down to 5°C, add m-chloroperoxybenzoic acid (0.8g, 3.9mmol) in batches, after addition, react at 0-10°C for 2 Hour. After the reaction was completed, the reaction solution was washed with sodium thiosulfate solution, and the layers were separated. Tetrahydrofuran was distilled off under reduced pressure to obtain a light yellow oil;
[0046] (3) Add tetrahydrofuran (15ml) to the light yellow oil in step (2), stir to dissolve, add 5-difluoromethoxy-2-mercapto-1H-benzimidazole, add 20% sodium hydroxide aqueous solution (11ml), after the addition, react at 20-30°C for 2 hours. After the reaction was completed, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com