Polyimide containing benzoxazole and carbazole structures as well as preparation method and application of same
A polyimide and benzoxazole technology, applied in the field of material science, can solve the problem of reducing the number of charge transfer complexes, and achieve the effects of reducing the charge transfer effect, high luminous intensity, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Wherein, the preparation method of the group Y of the diamine compound containing carbazole and benzoxazole structure shown in general structural formula Y-1 or Y-2 comprises the following steps:
[0046] (1) Utilize substituted p-fluorophenylboronic acid and 2-chlorobenzoxazole to couple through Suzuki reaction, obtain 2-substituted phenylbenzoxazole structure, its general structural formula is as structural formula (2):
[0047]
[0048] (2) Utilize the active hydrogen grafting step (1) in the dihalogenated carbazole monomer carbazole to obtain a dihalogenated structure containing a carbazole benzoxazole structure, and its general structural formula is as structural formula (3);
[0049]
[0050] Where X can be fluorine, chlorine, bromine or iodine.
[0051] (3) The dihalogenated structure obtained in step (2) and 4-aminophenylboronic acid or 3-aminophenylboronic acid are prepared by Suzuki coupling reaction to prepare diamine, wherein, reacting with 4-aminophen...
Embodiment 1
[0058] (1) Preparation of diamine monomer
[0059] a. Synthesis of intermediate 2-(4-fluorophenyl)-benzoxazole
[0060] Add 7.65g (50mmol) of 2-chlorobenzoxazole, 7.0g (50mmol) of p-fluorophenylboronic acid, and 10.28g of potassium carbonate (75mmol) into a 250mL three-necked round-bottomed flask, and add 100mL of tetrahydrofuran solution and 50mL of deionized water , magnetically stirred and introduced argon gas, then added 0.05g tetrakistriphenylphosphine palladium, raised the temperature to 90°C and stirred for 12 hours, then cooled, poured the reaction solution into water for precipitation, extracted with ethyl acetate, and separated the layers to obtain an organic layer , spin-dried, and purified by column chromatography to obtain 7.67 g of white product 2-(4-fluorophenyl)-benzoxazole, with a yield of about 72%, and its structural formula is as follows:
[0061]
[0062] b. Synthesis of intermediate substituted dibromocarbazole derivatives
[0063] Add 6.39g (30mmol)...
Embodiment 2
[0075] (1) Synthesis of meta-substituted target diamine monomer 2
[0076] 5.18g (10mmol) of substituted dibromocarbazole derivatives prepared in step b of Example 1, 6.64g (24mmol) of potassium carbonate and 6.58g (24mmol) of 3-aminophenylboronic acid were added to a 250mL double-necked round-bottomed flask, Add 80mL tetrahydrofuran, 40mL deionized water, magnetically stir and pass through argon protection, then add a catalytic amount of tetrakistriphenylphosphine palladium, heat up to 90°C and react for 24h, cool to room temperature, pour the reaction solution into water for precipitation, After filtration, it was fully washed with methanol, and after drying, it was purified by column chromatography to obtain 4.07 g of the target diamine monomer with a yield of about 75%. Its structural formula is as follows:
[0077]
[0078] (2) At -10°C, add 2.1688g (4mmol) of the diamine monomer prepared in step (1), 3.2038g (16mmol) of 4,4'-diaminodiphenyl ether and 24.4mL of N-N dim...
PUM
| Property | Measurement | Unit |
|---|---|---|
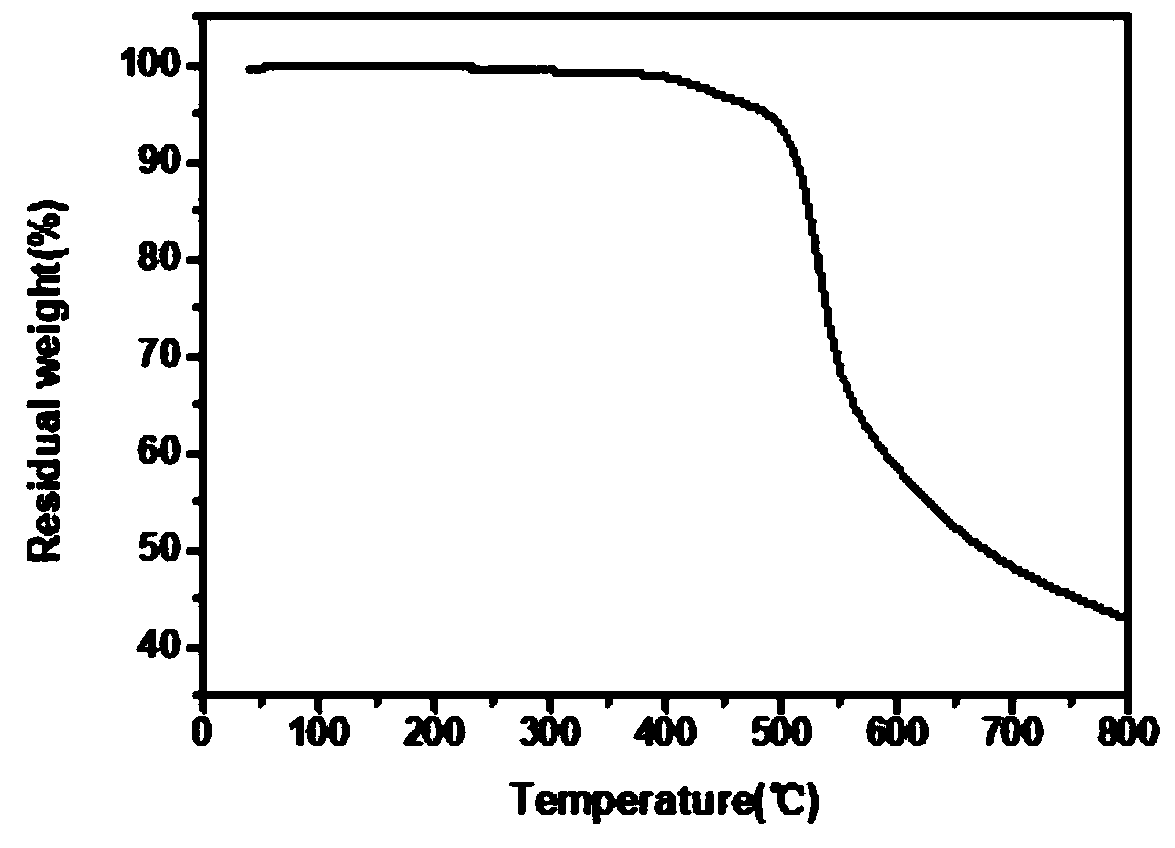
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com