A kit and method for efficient and rapid detection and quantification of serum or plasma nucleic acid
A technology of a kit and a nucleic acid releasing agent, which is applied to kits and fields for efficient and rapid detection and quantification of serum or plasma nucleic acids to achieve accurate results, accurate detection results, and fast speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
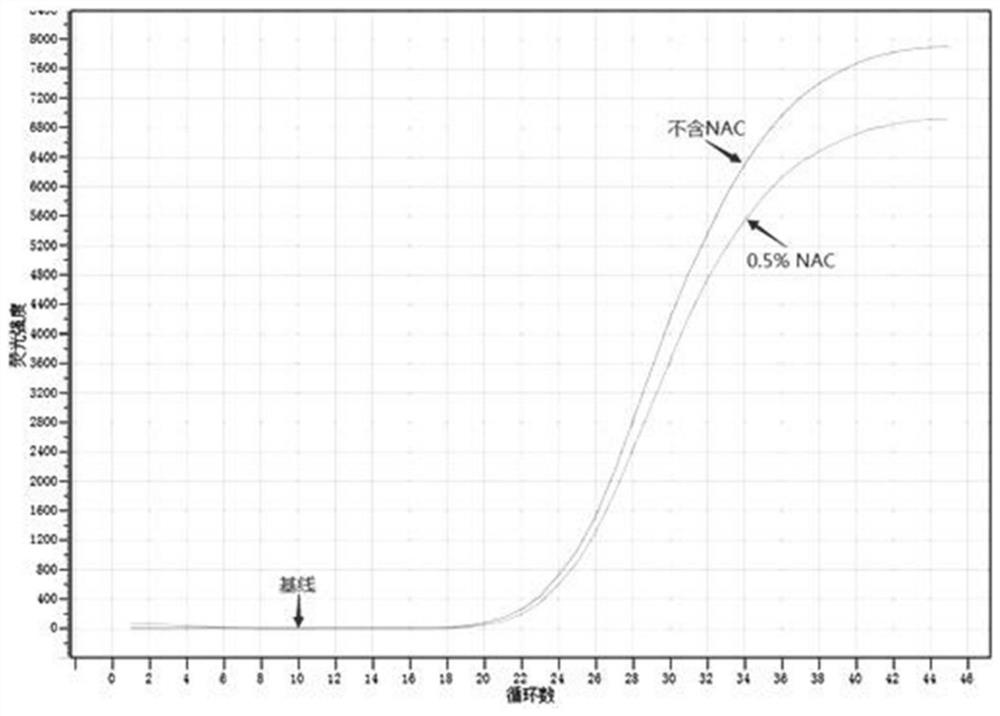
[0060] Example 1 Evaluation of adding N-acetyl cystine (NAC) to nucleic acid releasing agent
[0061] (1) Preparation of nucleic acid release agent A (without NAC): composed of 600 mM KOH, 0.06% (w / v) LLS, 2% (v / v) Tween 20, and the solvent is deionized nuclease-free water.
[0062] (2) Preparation of nucleic acid release agent B (containing 0.5% NAC): 600 mM KOH, 0.06%-0.1% (w / v) LLS, 0.05%-2% (v / v) Tween 20, 0.5% of NAC, and the solvent is nuclease-free deionized water.
[0063] (3) Preparation of reaction solution: the reaction solution is formulated as follows: 10 mM KCl, 5 mM MgCl 2 , 2mM EDTA-2Na, 25mM Tris-acetic acid, 10mM (NH 4 ) 2 SO 4 , BSA with a mass concentration of 0.02%, glycerol with a volume concentration of 8%, 1M betaine, trehalose with a mass concentration of 12%, 300nM upstream and downstream primers, 300nM probes, 1.5mM dNTPs, mixed The enzyme is composed of Proclin300 with a mass concentration of 0.03%, pH 8.0, and the solvent is deionized water w...
Embodiment 2
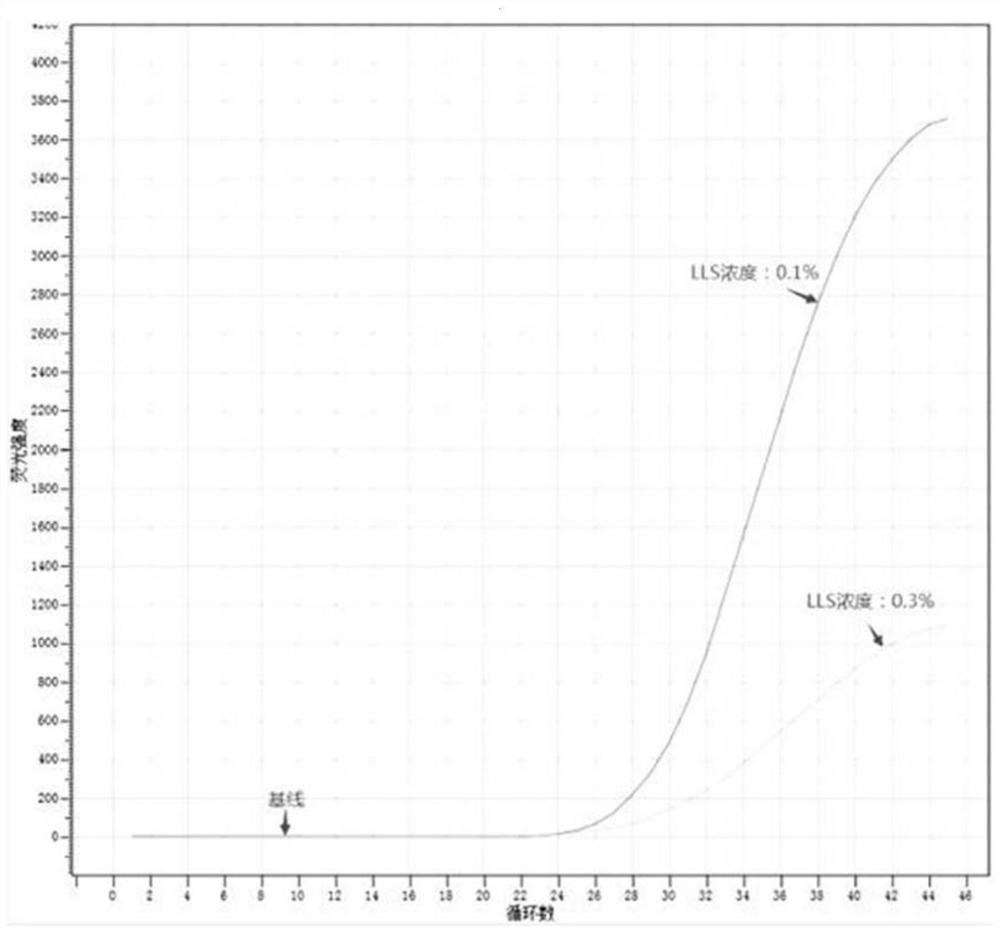
[0067] Example 2 Concentration evaluation of LLS (lithium dodecyl sulfate) in nucleic acid release agent
[0068] (1) Preparation of nucleic acid release agent A (0.1% LLS): composed of 600 mM KOH, 0.1% (w / v) LLS (lithium dodecyl sulfate, 2% (v / v) Tween 20), solvent It is nuclease-free deionized water.
[0069] (2) Preparation of nucleic acid release agent B (0.3% LLS): composed of 600 mM KOH, 0.3% (w / v) LLS (lithium dodecyl sulfate), 2% (v / v) Tween 20, The solvent was nuclease-free deionized water.
[0070] (3) Preparation of reaction solution: as in Example 1.
[0071] (4) Method: Two PCR reaction tubes, one of which was added with 5 μL of nucleic acid release agent A, and the other with 5 μL of nucleic acid release agent B, and then 5 μL of HBV DNA positive serum was added to the two PCR reaction tubes, respectively. Repeatedly pipetting for 5-7 times, mix well, and let stand at room temperature for 2 minutes; then add 40 μL of reaction solution to the two PCR tubes resp...
Embodiment 3
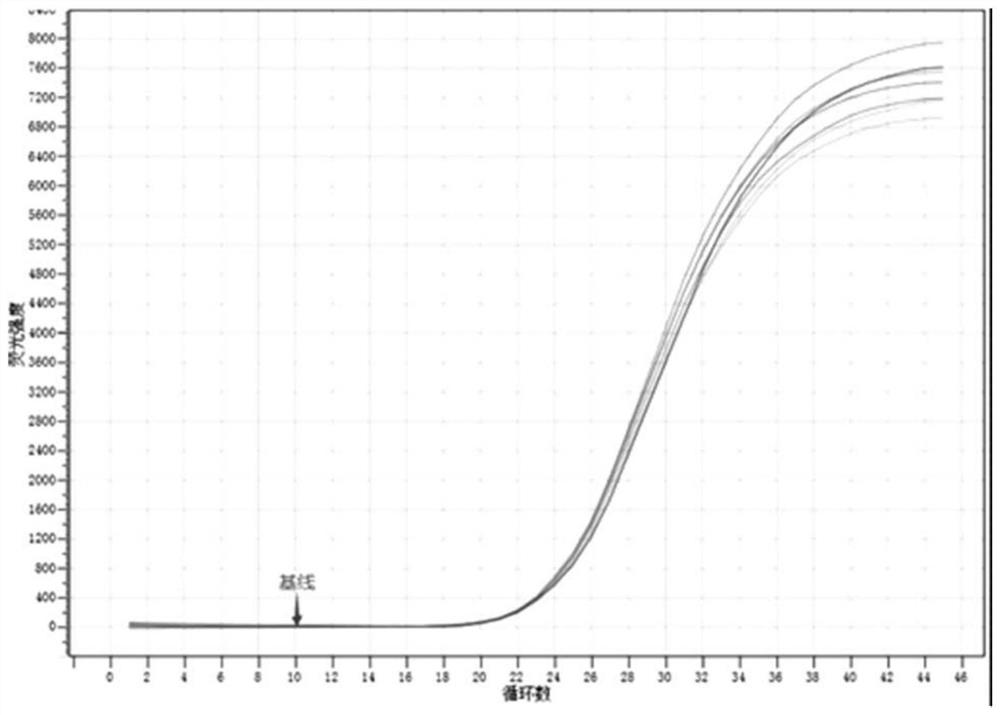
[0075] Example 3 Evaluation of the concentration of KOH in the nucleic acid releasing agent (template is DNA)
[0076] (1) Preparation of 7 kinds of nucleic acid release agents with different KOH concentrations (KOH concentrations are 300mM\350mM\400mM\450mM\500mM\550mM\600mM): 300mM~600mM KOH, 0.06% (w / v) LLS, 2% (v / v) Tween 20 in nuclease-free deionized water.
[0077] (2) Preparation of reaction solution: as in Example 1.
[0078] (3) Method: Add 5 μL of 7 kinds of nucleic acid release agents with different KOH concentrations into 7 PCR reaction tubes respectively, and then add 5 μL of HBV DNA positive serum to the 7 PCR reaction tubes respectively, mix well, and let stand at room temperature for 2 minutes; then add 40 μL of the reaction solution to the 7 PCR tubes respectively; finally mix and centrifuge, place the 7 PCR reaction tubes on the fluorescence quantitative PCR amplifier, select the FAM fluorescence channel, and set the amplification program to carry out the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com