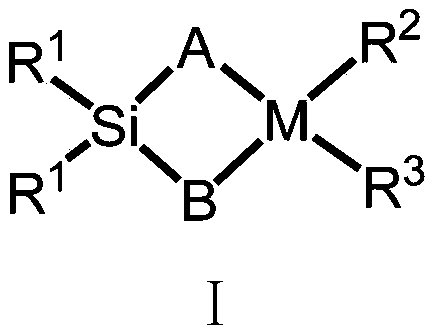
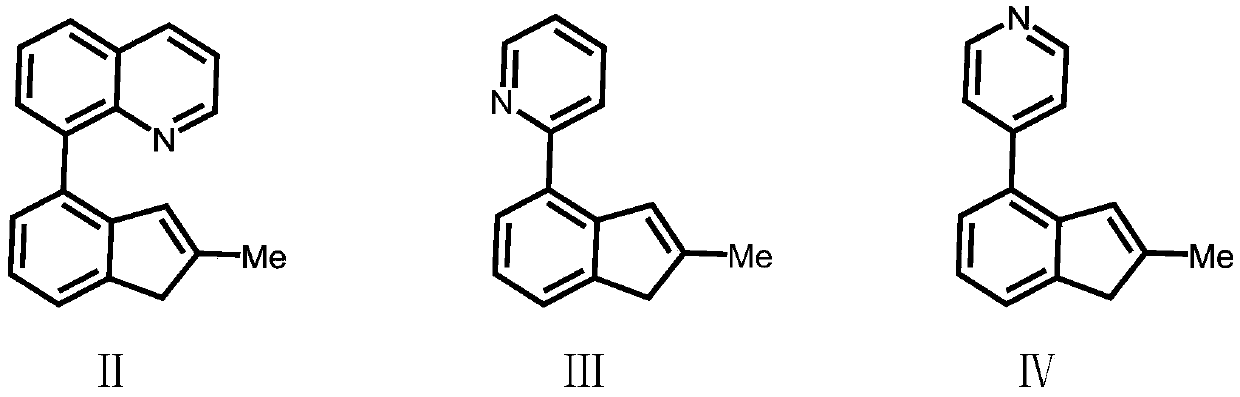
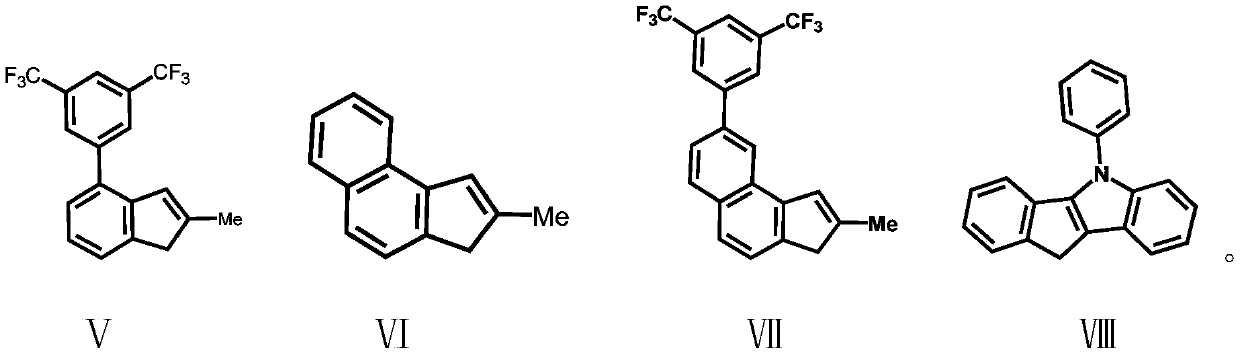
Silicon-bridged metallocene complex containing nitrogen heterocyclic ring structures and application thereof
A technology of metallocene complexes and silicon bridging, applied in metallocene, organic chemistry, chemical instruments and methods, etc., can solve the problem of low isotacticity of polypropylene, achieve simple preparation, good controllability, and promising application prospects broad effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Compound Formula Ⅱ: Preparation of 2-methyl-4-8-quinoline-indene
[0047] 8-Bromoquinoline (2.08g / 10mmol) was dissolved in 50mL of anhydrous tetrahydrofuran, and n-butyllithium (2.5M, 4mL / 10mmol) was added dropwise at -78°C, gradually raised to room temperature, and stirred at room temperature for 16 hour, trimethyl borate (1.04g / 10mmol) was added dropwise at -78°C, gradually warmed to room temperature, stirred at room temperature for 16 hours, water was gradually added at 0°C, and then 3.4mL of 3M aqueous hydrochloric acid was added dropwise. Extract with ether, adjust the pH to neutral with sodium bicarbonate, and recrystallize from acetone and n-hexane to obtain intermediate product 1 (1.5 g, yield 87%). Intermediate product 1 structural formula:
[0048]
[0049] The obtained intermediate product 1 (1.72g / 10mmol), 2-methyl-4-bromoindanone (1.46g / 10mmol), 0.005mol% Pd(OAc) 2 Dichloromethane solution, tetrabutylammonium bromide (3.22g / 10mmol), potassium carbonate...
Embodiment 2
[0054] Preparation of Ligand L1(2-methyl-4-8-quinoline-indene)(2-methyl-4-3,5-ditrifluoromethyl-phenyl-indene)dimethylsilyl
[0055] Under a nitrogen atmosphere, dissolve the compound formula II (2.57g / 10mmol) in 50mL of anhydrous tetrahydrofuran, add n-butyllithium (2.5M, 4mL / 10mmol) dropwise at -78°C, and gradually rise to Return to room temperature, stir at room temperature for 16 hours, then add the lithium salt solution formed by the reaction into a tetrahydrofuran solution of dimethyldichlorosilane (50 mmol) at -78°C, gradually rise to room temperature and continue stirring for 16 hours. The solvent and excess dimethyldichlorosilane were removed under reduced pressure, and 50 mL of anhydrous tetrahydrofuran was added to the system. At -78 ° C, the compound formula V was also treated with n-butyllithium (2.5M, 4 mL / 10 mmol ) ionization, the tetrahydrofuran solution of the ionized compound V was added into the tetrahydrofuran solution of the above compound, and the mixtur...
Embodiment 3
[0057] Preparation of Ligand L2(2-methyl-4-8-quinoline-indene)(2-methyl-benzoindene)dimethylsilyl
[0058] The experimental procedure was the same as in Example 2, and 4.55 g, 9.22 mmol of ligand L2 was obtained, yield: 92%. Anal. Calcd for C 39 h 31 f 6 NSi: C, 71.43; H, 4.77; N, 2.14. Found: C, 70.83; H, 5.34; N, 2.01.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com