Application of exosome in preparation of anti-infective drugs
An exosome, anti-infection technology, applied in the field of biomedicine, can solve the problems of difficult treatment, treatment failure drug resistance, drug resistance and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
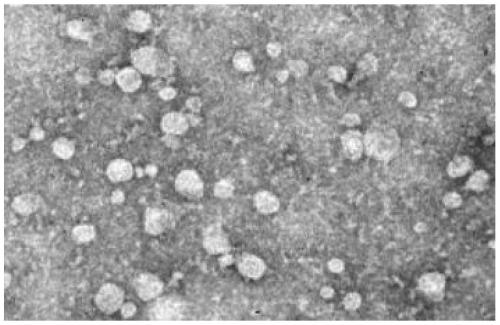
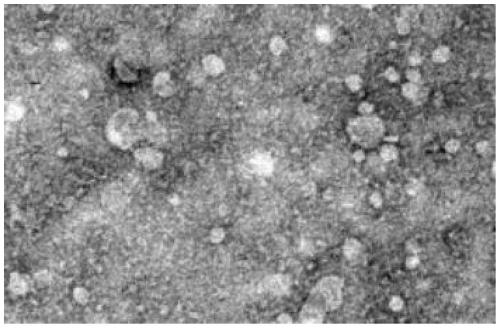
[0023] Example 1: Culture of RAW264.7 cells and extraction and identification of exosomes
[0024] 1. Experimental materials
[0025] DMEM high-glucose medium was purchased from Hyclone Company; fetal bovine serum was purchased from Gibco Company; mouse macrophage RAW264.7 was purchased from Shanghai Cell Bank of Chinese Academy of Sciences; Exosome Rapid Extraction Reagent (ExoQuick) was purchased from SBI Company.
[0026] 2. Isolation and Characterization of Exosomes
[0027] RAW264.7 cells were cultured with 10% volume fraction of exosome-free fetal bovine serum DMEM medium + mass fraction of 1% penicillin-streptomycin complete medium. The cell culture fluid was collected, and exosomes were extracted according to the instructions of the ExoQuick kit. The specific steps are as follows: transfer the culture supernatant to a centrifuge tube, and centrifuge to remove cell debris. Take the supernatant and transfer it to a new centrifuge tube, add the reagent according to the...
Embodiment 2
[0031] Example 2: Encapsulation of Linezolid (LZD) by co-incubation
[0032] Quantitative standard curve determination of LZD: Accurately weigh LZD powder and dissolve it in DMSO to make a stock solution of 10 mg / mL, and store it at -80°C after aliquoting. Precisely draw an appropriate amount of LZD stock solution, dilute with acetonitrile to make standard solutions with final concentrations of 0.5, 1, 2, 4, 10, 20, 40 μg / mL, and inject 50 μL. Chromatographic conditions: Chromatographic column: Extend-C18, 250mm×4.6mm, 5μm, Agilent; mobile phase: acetonitrile: water = 20:80; flow rate: 1 mL / min; column temperature: 30° C.; detection wavelength: 251 nm. After obtaining the corresponding experimental results, linear regression is performed with the concentration of the analyte as the ordinate and the peak area of the analyte as the abscissa, and the linear regression equation is obtained: Y=187.45x+10.227, R 2 =1, the linear relationship is good, and its linear range is 0...
Embodiment 3
[0035] Example 3: Application of linezolid-loaded exosomes (ExoLZD) to treat MRSA infection in RAW264.7 cells
[0036] RAW264.7 intracellular MRSA infection model construction: RAW264.7 cells were completely cultured with DMEM with a volume fraction of 10% fetal bovine serum at 37°C, 5% CO2 cultured in an incubator. Bacterial MRSA was cultured to the logarithmic phase, centrifuged at 4000rpm / min for 10min, discarded the supernatant, resuspended with the above-mentioned complete medium, and cultured again at 37°C for 30min. Replace the original macrophage culture medium with this bacteria-containing medium. After 2 hours of infection, wash the extracellular bacteria with sterile PBS buffer, and check whether the extracellular bacteria are eliminated by the live bacteria agar plate method.
[0037] Observation of ExoLZD treatment of MRSA infection in RAW264.7 cells by plate colony counting method: 2 hours after MRSA infected RAW264.7 cells, culture medium containing LZD, ExoLZD,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com