Preparation method for bis(3-amino-4-hydroxyphenyl)hexafluoropropane
A technology of hexafluoropropane and hydroxyphenyl, which is applied in the field of dihexafluoropropane preparation, can solve the problems of harsh reaction conditions, difficult wastewater treatment, high price, etc., and achieve simple separation and purification of products, mild reaction conditions, and less side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
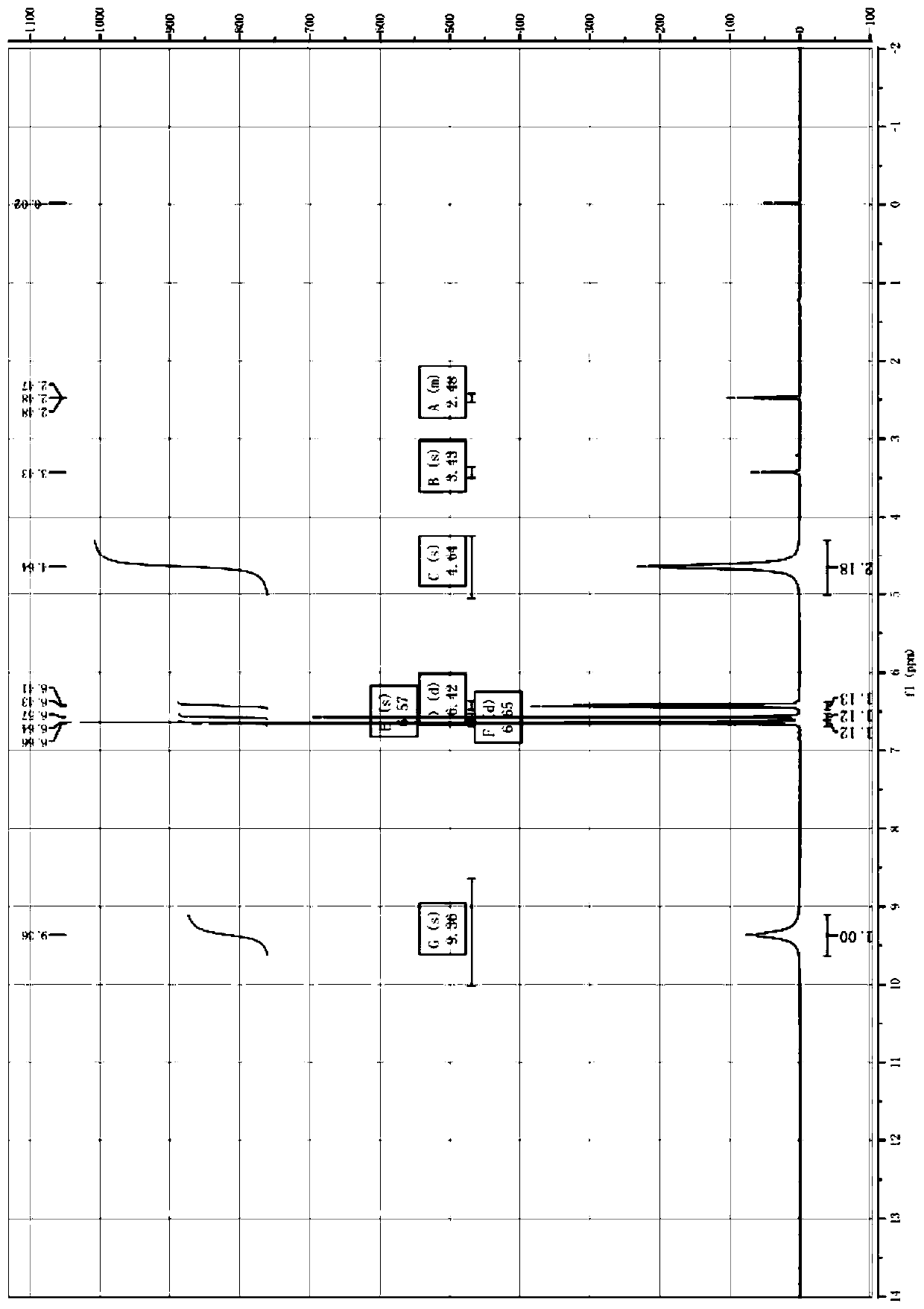
Image
Examples
preparation example Construction
[0024] In order to solve the above problems, the invention provides a kind of preparation method of two (3-amino-4-hydroxyphenyl) hexafluoropropane, comprising the following steps:
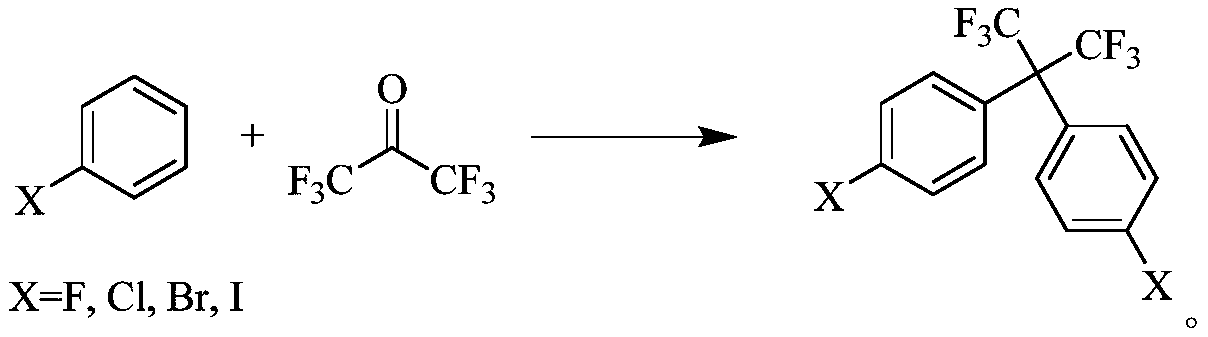
[0025] The first step: the reactant (I) is passed through hexafluoroacetone in the presence of a catalyst to react to obtain the compound (II);
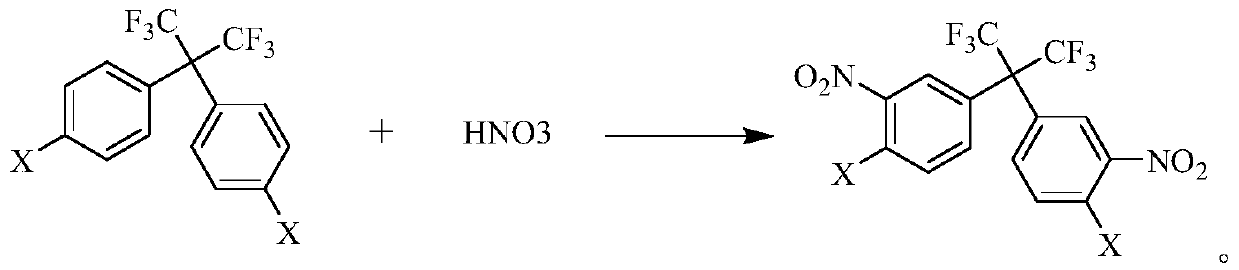
[0026] The second step: reacting the compound (II) obtained in the first step with nitric acid and sulfuric acid to obtain the compound (III);
[0027] The third step: adding the compound (Ⅲ) obtained in the second step to an inorganic base to react to obtain the compound (Ⅳ);
[0028] The fourth step: Catalytic hydrogenation of the compound (IV) obtained in the third step to obtain bis(3-amino-4-hydroxyphenyl)hexafluoropropane;
[0029] Wherein, the reactant (I) is a halogenated benzene, the compound (II) is bis(4-halophenyl)hexafluoropropane, and the compound (III) is bis(3-nitro-4-halophenyl)hexafluoropropane Fluoropropane, the compound (IV) is bis(...
Embodiment 1
[0088] Embodiment 1 provides a kind of preparation method of bis(3-nitro-4-fluorophenyl)hexafluoropropane, the synthesis reaction formula is as follows:
[0089]
[0090] Preparation steps include:
[0091] The first step: the synthesis of bis(4-fluorophenyl)hexafluoropropane
[0092] Put 96.1g of fluorobenzene into a 250mL four-neck bottle, add 20.7g of aluminum trichloride, start stirring and raise the temperature to 60°C, and pass hexafluoroacetone gas at a rate of 100mL / min. After aeration for 10 hours, the intermediate is controlled in the gas phase When the p-fluorophenylhexafluoroacetone content is 50%, stop ventilation, raise the kettle temperature to reflux reaction at 80-85°C, stop the reaction when the p-fluorophenylhexafluoroacetone content<1.0%, and the target product bis(4- The gas phase content of fluorophenyl)hexafluoropropane is 98%, and the system is black; after the kettle temperature is lowered to room temperature, 90g of water is added dropwise to quen...
Embodiment 2
[0096] Embodiment 2 provides a kind of preparation method of bis(3-nitro-4-chlorophenyl)hexafluoropropane, the synthesis reaction formula is as follows:
[0097]
[0098] Preparation steps include:
[0099] The first step: the synthesis of bis(4-chlorophenyl)hexafluoropropane
[0100] Put 133.8g of chlorobenzene into a 500mL four-necked bottle, add 36.0g of aluminum trichloride, start stirring and raise the temperature to 70°C, and pass in hexafluoroacetone gas at a speed of 100mL / min. After aeration for 6 hours, the intermediate p-chlorobenzene When the content of p-chlorophenyl hexafluoroacetone is 50%, stop the ventilation, raise the still temperature to react at 80-85°C, stop the reaction when the content of p-chlorophenyl hexafluoroacetone <1.0%, and the target product bis(4-chlorophenyl) The gas phase content of hexafluoropropane is 98%, and the system is black. Lower the temperature of the kettle to room temperature, add 100g of dichloromethane, slowly add 100g of w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com