Preparation method of hollow cerium dioxide
A ceria, hollow technology, applied in the field of preparation of hollow ceria, can solve the problems of long period, cumbersome process of hollow ceria, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] A kind of preparation method of hollow ceria provided by the invention comprises the following steps:
[0027] In step S1, water-soluble trivalent cerium salt and citric acid and / or citrate are dissolved in deionized water to form a mixed solution, and Ce in the mixed solution 3+ The ratio of the amount of the substance to the citrate ion is 1:1-2:1.
[0028] Further, in this step, the water-soluble trivalent cerium salt may be cerium nitrate or cerium chloride.
[0029] Preferably, Ce in the mixed solution 3+ The concentration is 0.3mol / L~0.9mol / L.
[0030] Further, in this step, the citrate may be sodium citrate.
[0031] Preferably, the concentration of citrate ions in the mixed solution is 0.2mol / L-0.5mol / L.
[0032] Citric acid and / or citrate as an organic linker is a polycarboxylic acid organic compound with carboxyl ligands; in addition, citric acid or citrate can be partially deprotonated or completely deprotonated with different pH In effect, there are mul...
Embodiment 1
[0041] First, 0.4130 g of cerium nitrate hexahydrate and 0.1010 g of citric acid monohydrate were dissolved in 2 mL of deionized water to prepare a mixed solution.
[0042] Then, the above mixed solution was placed in a polytetrafluoroethylene-lined autoclave and subjected to ultrasonic treatment for 20 min, and a hydrothermal reaction was performed at 210° C. for 1 h to obtain a hydrothermal product.
[0043] Finally, after the reaction was completed, the hydrothermal product was cooled to room temperature, and after solid-liquid separation, the obtained solid phase was centrifuged and washed three times with ethanol, and vacuum-dried for 2 hours to obtain the product.
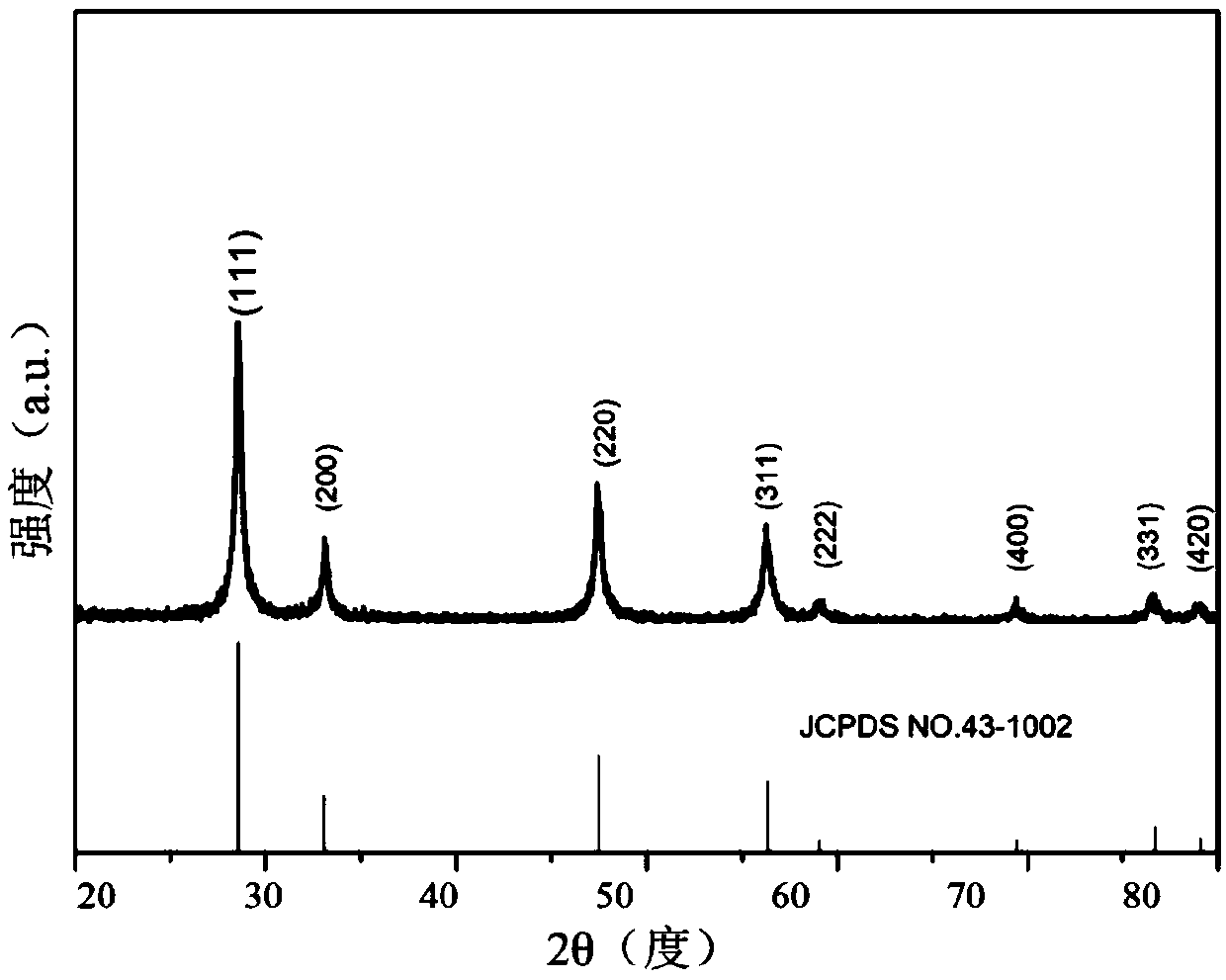
[0044] Adopt X-ray diffractometer (Germany Bruker D8 advance, Cu target radiation λ=0.15418nm) to measure the product that present embodiment obtains, its X-ray diffraction test (XRD) result is as follows figure 1 shown. The diffraction peaks of this product are similar to those of fluorite-type CeO 2 The d...
Embodiment 2
[0048] First, 2.4490 g of cerium nitrate hexahydrate and 0.5930 g of citric acid monohydrate were dissolved in 10 mL of deionized water to prepare a mixed solution.
[0049] Then, the above mixed solution was placed in a polytetrafluoroethylene-lined autoclave and subjected to ultrasonic treatment for 20 min, and a hydrothermal reaction was performed at 230° C. for 5 h to obtain a hydrothermal product.
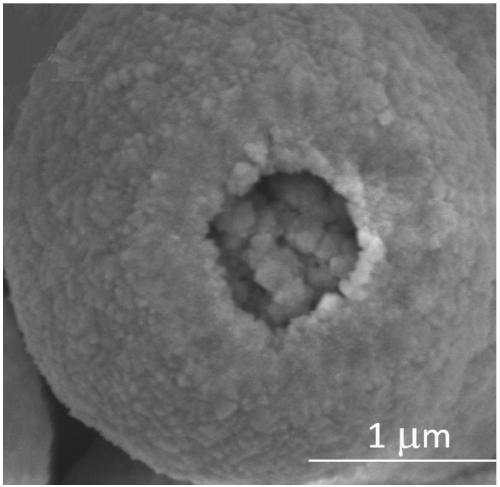
[0050] Finally, after the completion of the hydrothermal reaction, the hydrothermal product is cooled to room temperature, and after solid-liquid separation, the obtained solid phase is centrifuged and washed three times with deionized water, and vacuum-dried for 8 hours to obtain hollow cerium oxide. The outer diameter of the hollow cerium oxide is is 3 μm, and the shell thickness is 100 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| Shell thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com