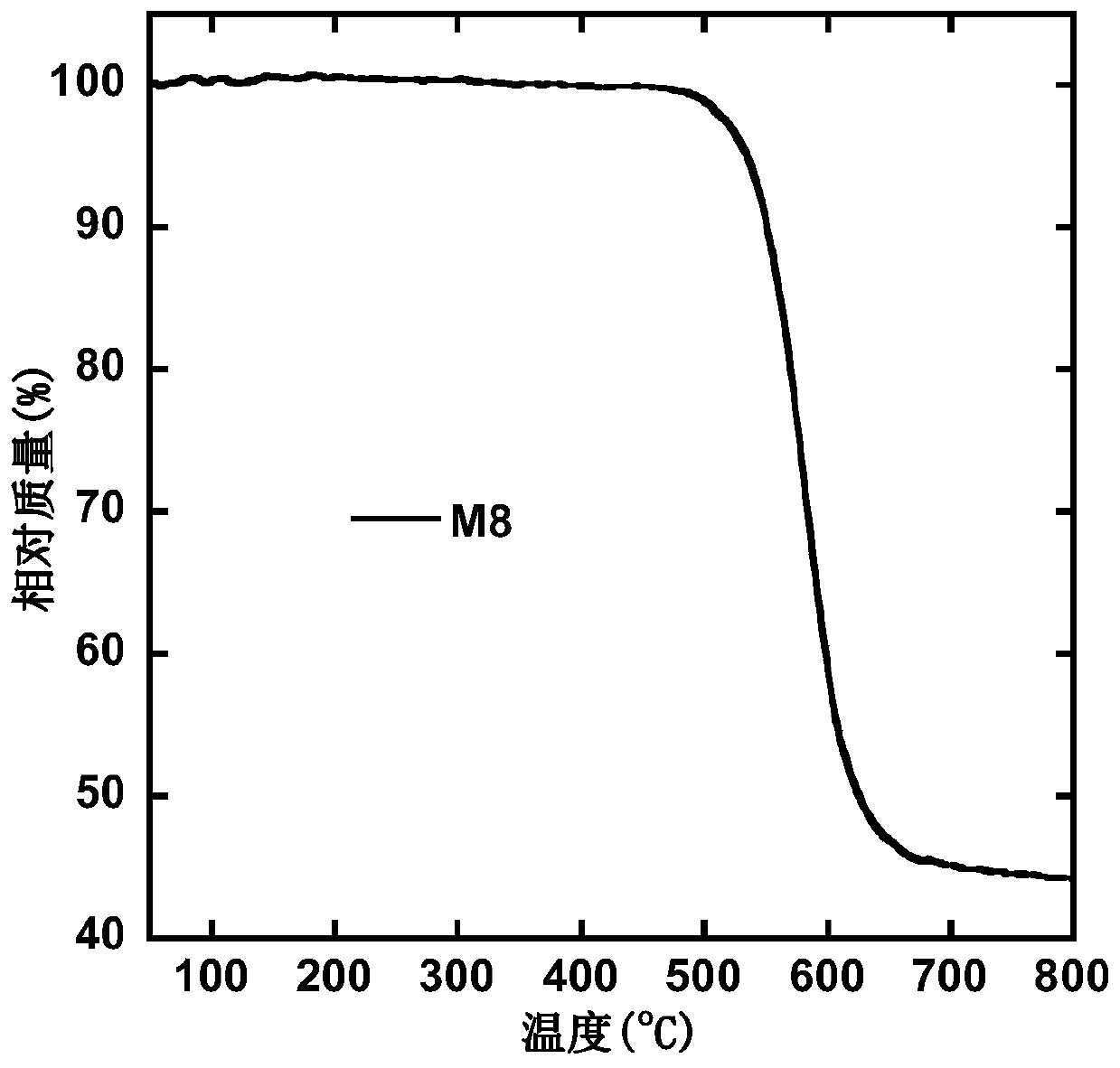
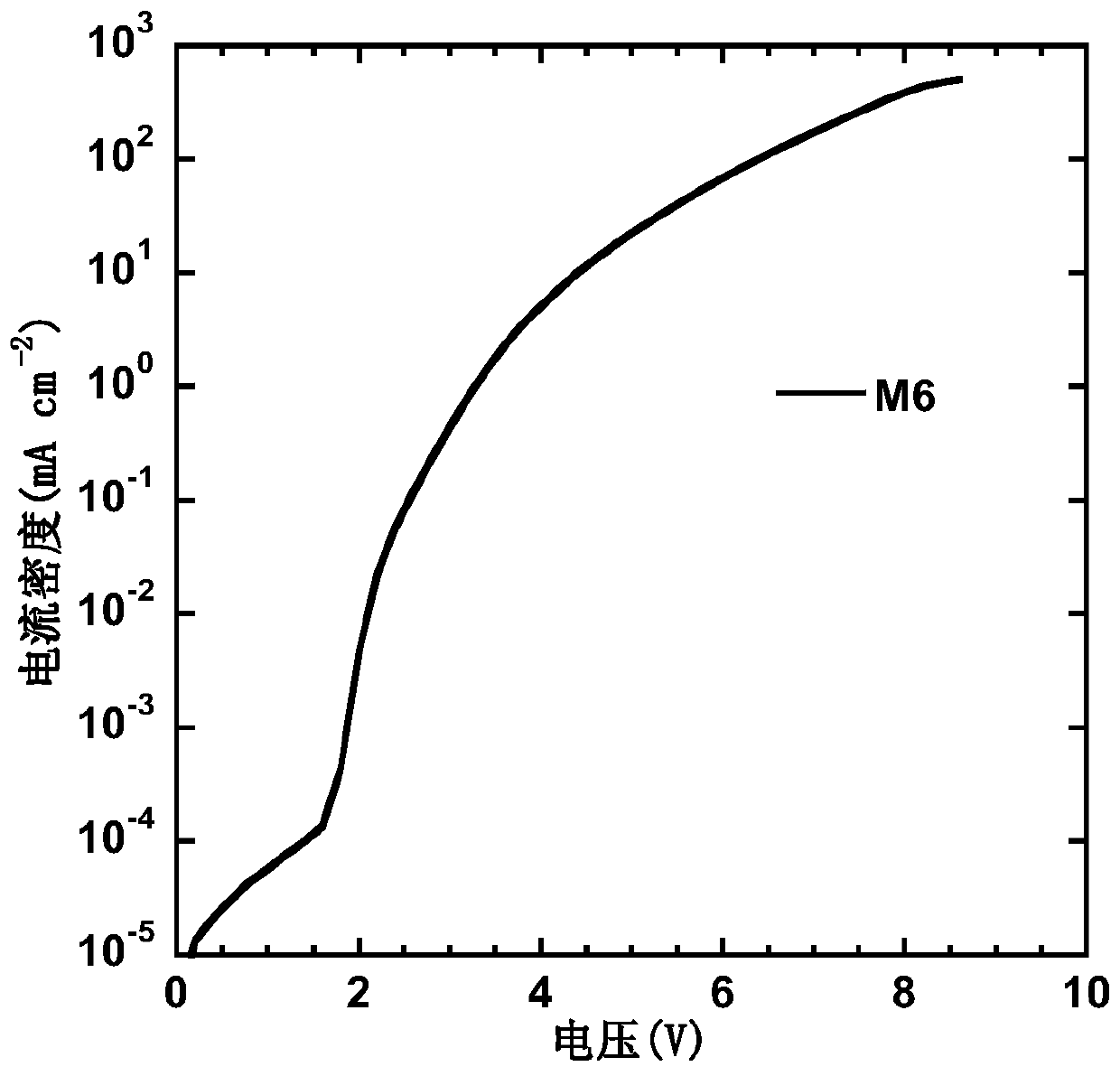
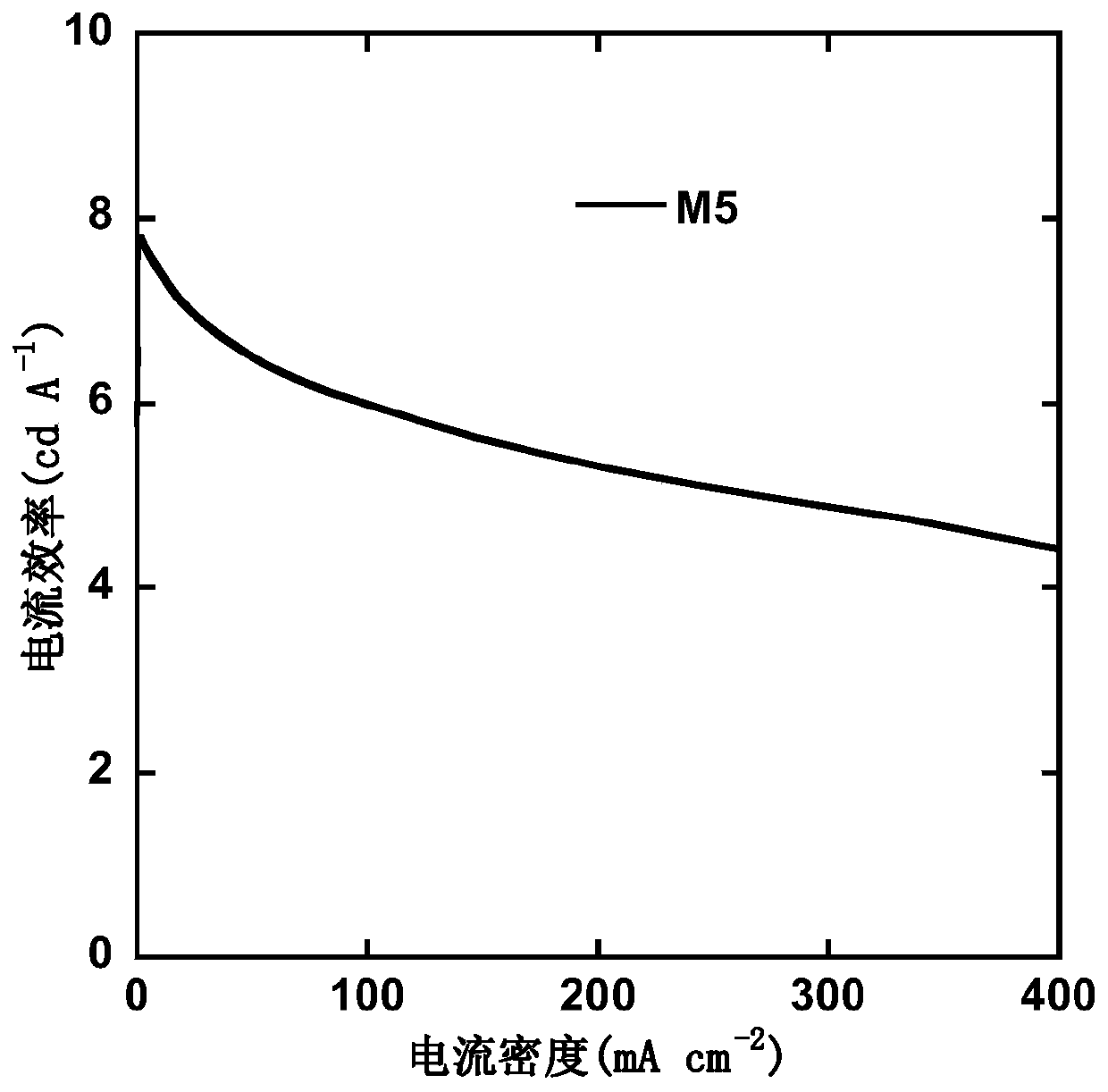
S,S-dioxo-dibenzothiophene and phenanthroimidazole based small molecule and application thereof in electroluminescent devices
A technology of dibenzothiophene and phenanthroimidazole, which is applied in the field of organic photoelectric materials, can solve the problems of failure to attract widespread attention and high device driving voltage, and achieve good hole and electron injection and transport capabilities, good thermal stability, Effect of High Fluorescence Quantum Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of compound M1
[0025] The structural formula and synthetic route of compound M1 are shown below, and the specific synthetic method is as follows
[0026]
[0027] (1) Synthesis of compound 1
[0028] Dissolve dibenzothiophene (10 mmol) in 100 mL of anhydrous tetrahydrofuran, cool to -78°C, add n-butyllithium solution (concentration: 2M, 5 mL) dropwise, raise to 0°C and stir for 2 hours after the addition is complete. Then the temperature was lowered to -78°C, 1,2-dibromoethane (15 mmol) was added to the reaction solution, and then the mixture was naturally raised to room temperature for 12 hours. After the reaction was completed, the reaction was quenched with 2 ml of deionized water, and the tetrahydrofuran was distilled and spin-dried under reduced pressure to obtain a crude product. Recrystallization with tetrahydrofuran / ethanol mixed solvent gave a white solid product with a yield of 60%.
[0029] (2) Synthesis of compound 2
[0030] Compound 2 ...
Embodiment 2
[0038] Preparation of compound M2
[0039] The structural formula and synthetic route of compound M2 are shown below, and the specific synthetic method is as follows
[0040]
[0041] (1) Synthesis of compound 5
[0042] Under nitrogen protection, phenanthrenequinone (10mmol), p-bromobenzaldehyde (10mmol) and aniline (10mmol) were added to 90ml of acetic acid, and heated to reflux for 24 hours. After cooling to room temperature and standing still, suction filtration was performed, and the filter residue was washed with ethanol three times to obtain a crude product. Recrystallization with tetrahydrofuran / ethanol mixed solvent gave a white solid product with a yield of 89%.
[0043] (2) Synthesis of compound 6
[0044] In a nitrogen atmosphere, reactant 5 (10mmol), biboronic acid pinacol ester (12mmol), potassium acetate (10mmol), Pd(dppf)Cl 2 (0.5mmol) was added to the reaction and dissolved with 100mL 1,4-dioxane, heated to 80°C and reacted for 12 hours. After the reacti...
Embodiment 3
[0048] Preparation of compound M3
[0049] The structural formula and synthetic route of compound M3 are shown below, and the specific synthetic method is as follows
[0050]
[0051] (1) Synthesis of Compound 7
[0052] Add S,S-dioxy-dibenzothiophene (10mmol) and concentrated sulfuric acid (50ml) into a 100ml two-necked bottle, and add N-bromosuccinyl in three batches under nitrogen protection and dark conditions. Amine (10 mmol), stirred at room temperature for 24 hours. The reaction solution was slowly poured into 1000ml of ice water, filtered, and the filter residue was washed three times with distilled water, and then washed three times with ethanol. The crude product was filtered and recrystallized three times with chlorobenzene to obtain a white solid with a yield of 66%. 1 H NMR, 13 The results of C NMR, MS and elemental analysis showed that the obtained compound was the target product.
[0053] (2) Synthesis of compound M3
[0054] Under the protection of nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com