Preparation method and application of polypropylene oxide-sodium alginate hydrogel
A technology of polypropylene oxide and sodium alginate, applied in the field of medicine, can solve the problems of short duration of effective concentration, inability to meet clinical treatment, etc., and achieves the improvement of action time and treatment effect, high drug loading efficiency, and improved treatment effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthetic method of polypropylene oxide-sodium alginate hydrogel:
[0022] Formula (III) (8.41 g, 40 mmol), formula (II) (1.93 g, 5 mmol) and 4-dimethylaminopyridine (0.0386 g, 0.316 mmol) were weighed and dissolved in 20 mL of anhydrous tetrahydrofuran. Then, dicyclohexylcarbodiimide (3.09 g, 15 mmol) was added into anhydrous tetrahydrofuran (5 mL), and dropped into an ice bath. The resulting mixture was stirred at 30 °C for 12 h, the reactant was filtered, the solvent was removed by rotary evaporation, and the product was purified by recrystallization from ethyl acetate to obtain the product formula (IV);
[0023] Weigh 2g formula (Ⅳ) (3.5mmol) and 1.32g EDC (6.9mmol) and dissolve in 20mL absolute ethanol, stir and react for 50 minutes to activate the carboxyl group; formula (Ⅴ) (3.5mmol) and 0.079g NHS (0.687mmol) Dissolve in 20mL of deionized water to activate the hydroxyl group, and slowly add the activated formula (Ⅴ) (3.5mmol) into the aqueous solution of th...
Embodiment 2
[0025] Preparation of Nedaplatin-loaded polypropylene oxide-sodium alginate hydrogel
[0026] Nedaplatin was encapsulated into the crosslinked network of polypropylene oxide-sodium alginate hydrogel. 50mg of polypropylene oxide-sodium alginate hydrogel, 10mg of nedaplatin were dissolved in the dimethyl sulfoxide / water system with a volume ratio of 1:9, and after desalting at room temperature for 24 hours, it was slowly added dropwise into a large amount of ultrapure water (500 mL), and then stirred rapidly at room temperature for 1 hour. The solution was dialyzed against ultrapure water and filtered through a 0.45 μm needle filter to obtain nedaplatin-loaded hydrogel.
Embodiment 3
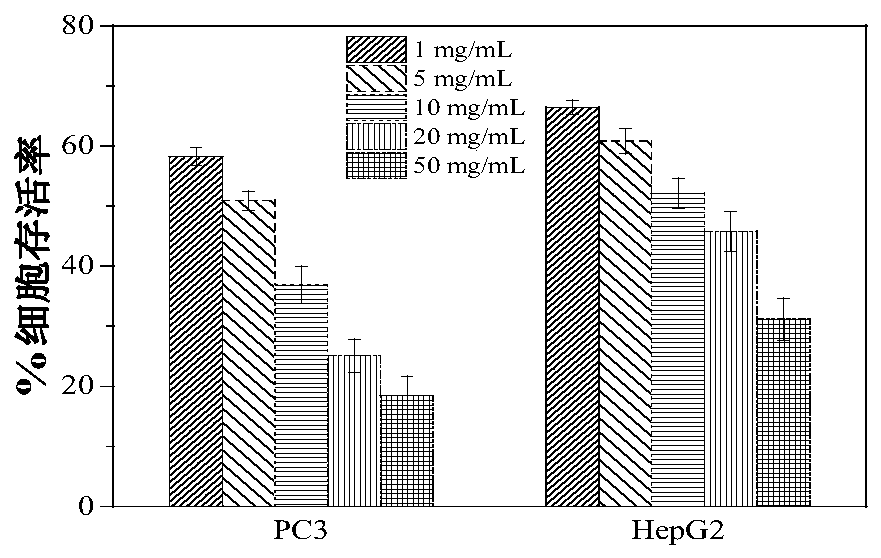
[0028] Drug loading capacity (DLC) and drug loading efficiency (DLE) of nedaplatin-loaded polypropylene oxide-sodium alginate hydrogel
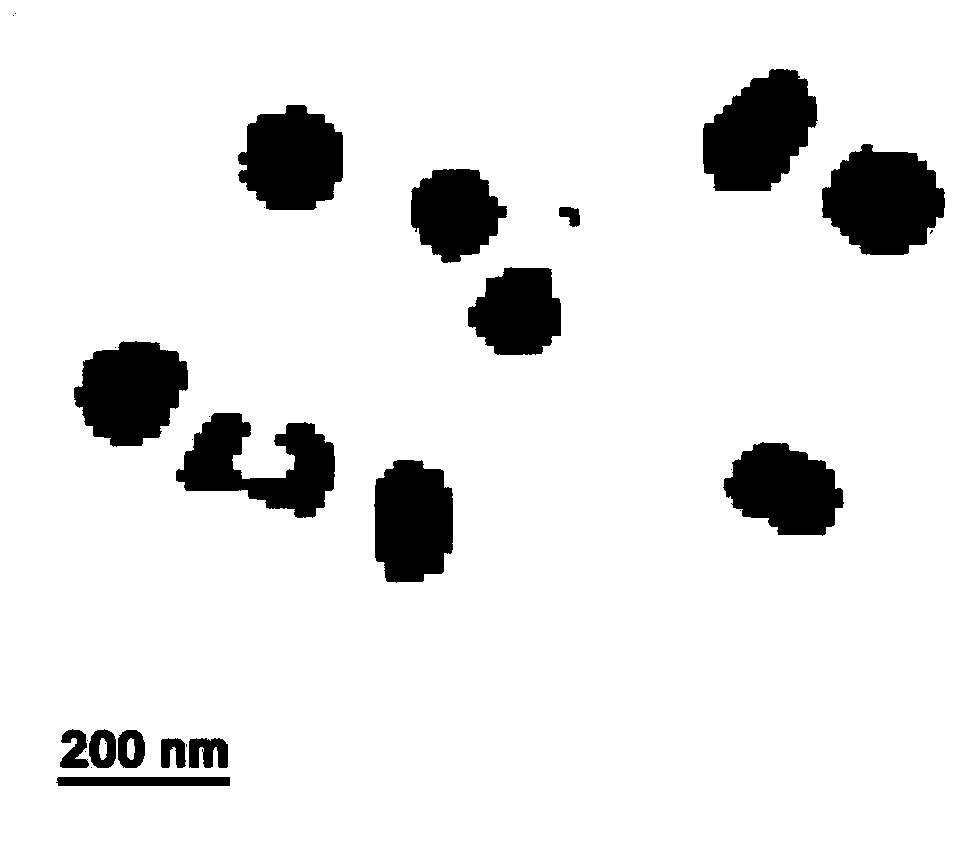
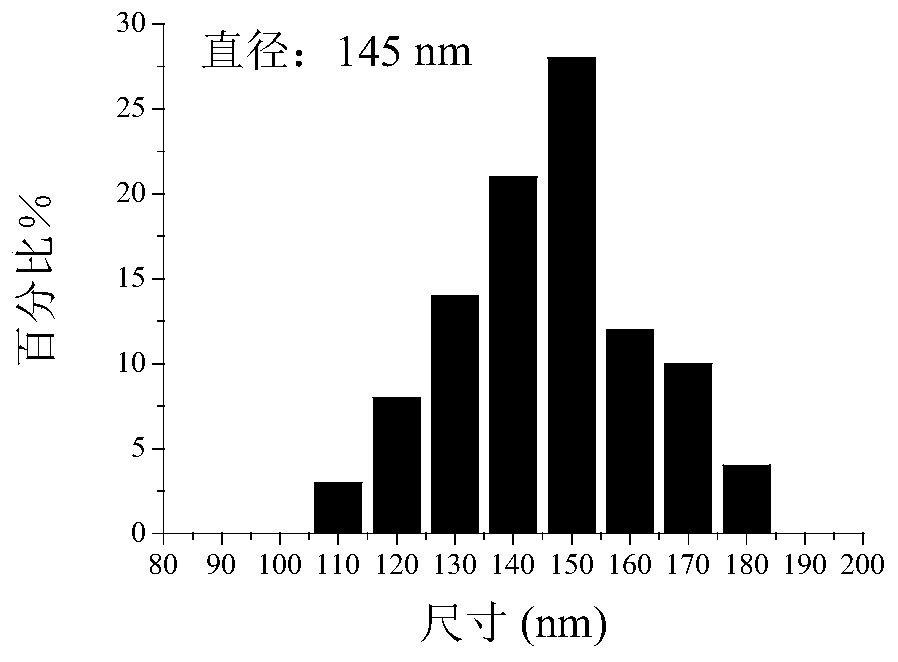
[0029] Morphology: A drop of hydrogel solution was evenly dropped on the copper grid with carbon film, and then after it was dried at room temperature, TEM was used to capture the topography picture of the micelles. Particle size: The average size and size distribution of the micelles were determined by DLS. figure 1 The topography images of chitosan-sodium alginate hydrogels loaded with nedaplatin were captured by TEM, figure 2 Size distribution profile of nedaplatin-loaded polypropylene oxide-sodium alginate hydrogels determined by DLS.
[0030] Drug Loading Capacity (DLC) and Drug Loading Efficiency (DLE): Freeze Nedaplatin-loaded polypropylene oxide-sodium alginate hydrogel to obtain a blue solid powder, dissolve the powder in methanol, and repeat the preparation of 4 batches Nedaplatin-loaded polypropylene oxide-sodium alginate hydrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com