640nm excited near-infrared fluorescent dye and preparation method thereof
A fluorescent dye and near-infrared technology, applied in the field of fluorescent dyes, can solve the problems of few near-infrared dyes and unclear structure-activity relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
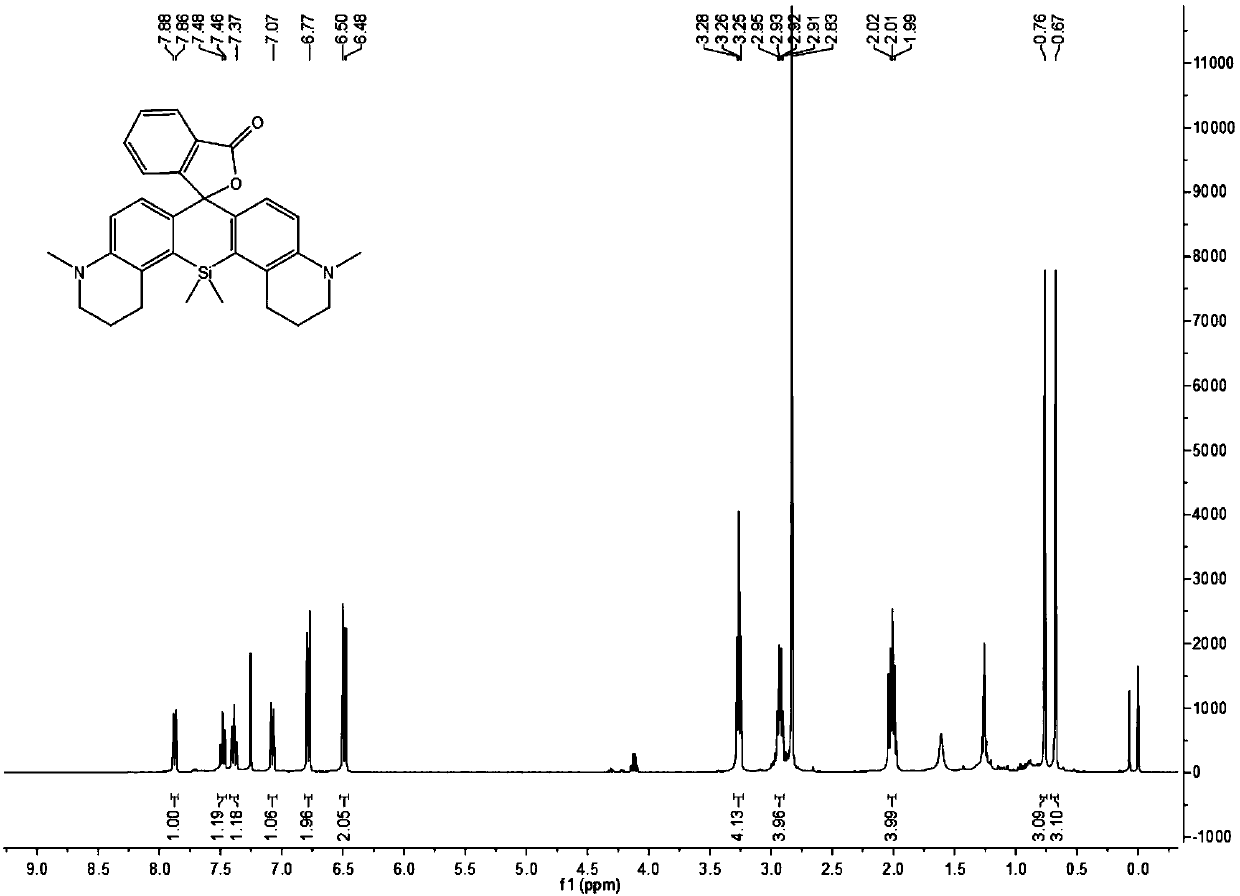
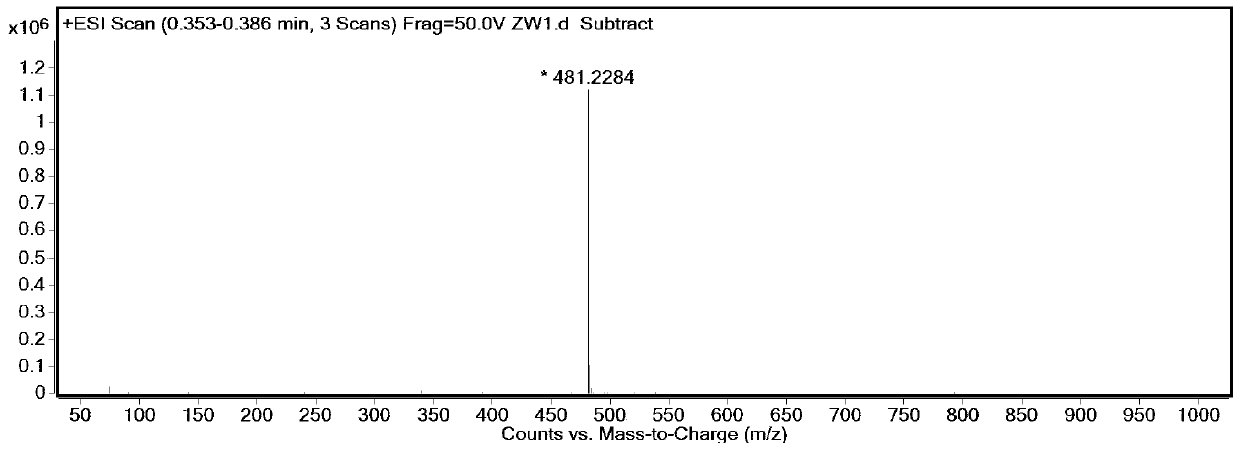
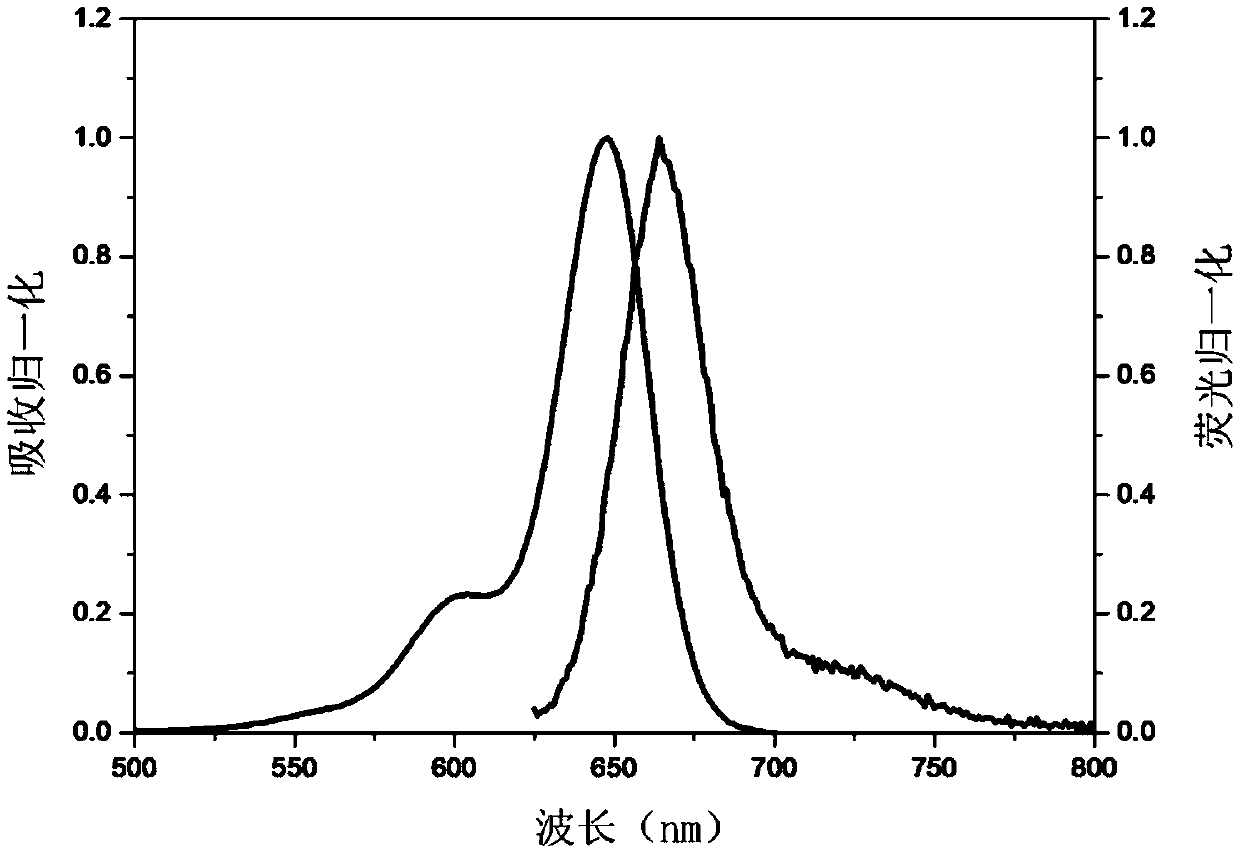
[0071] Synthesis of target fluorescent dye SiR-1
[0072] Synthesis of Intermediate 5-Bromotetrahydroquinoline
[0073]
[0074] 5-Bromoquinoline (3.5g) was dissolved in 45mL of acetic acid, under the condition of ice bath, sodium cyanoborohydride was added in a small amount in batches, a total of 2.5g, raised to room temperature and stirred for 2h, then added paraformaldehyde and continued to stir for 4h Finally, most of the acetic acid was removed under reduced pressure, the pH was adjusted to be weakly alkaline with saturated aqueous sodium carbonate solution, extracted with ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, and the organic solvent was removed under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column with pure petroleum ether as the eluent. After removal of the organic solvent, 1.7 g of a colorless liquid was obtained, with a yield of 46%. Its nuclear magnetic spectrum hydr...
Embodiment 2
[0098] Synthesis of target fluorescent dye SiR-2
[0099] Synthesis of intermediate 5
[0100]
[0101] 3.5g of 4-bromoindole was dissolved in 45mL of acetic acid, and sodium cyanoborohydride was added in small amounts in batches under ice-bath conditions, totaling 2.2g, raised to room temperature and stirred for 4 hours, then the reaction was stopped. After most of the acetic acid was removed under reduced pressure, the pH was adjusted to weak alkalinity with saturated sodium carbonate solution, extracted three times with ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, and the organic solvent was removed to obtain 3.2 g of a colorless liquid product. The rate is 91%.
[0102] Synthesis of Intermediate 6
[0103]
[0104] Weigh 381 mg of 70% oily dispersion of sodium hydride in a Shrek bottle, under nitrogen protection and ice bath, add 1.0 g of 4-bromoindoline dissolved in tetrahydrofuran solution, 1.6 g of methyl iodide, and stir ...
Embodiment 3
[0128] Synthesis of target fluorescent dye SiR-3
[0129] Synthesis of intermediate 10
[0130]
[0131] Dissolve 270 mg of N-methyl-4-bromoindoline in 5 mL of DMF, add 140 μL of phosphorus oxychloride, raise the temperature to 80-100 ° C and stir, adjust the acidity and alkalinity to weak alkalinity after cooling, extract with dichloromethane, and wash After drying and removing the organic solvent under reduced pressure, add 10 mL of methanol, slowly add 40 mg of sodium borohydride, and stir at room temperature for 1-4 h. After methanol was removed under reduced pressure, it was quenched with water, extracted with dichloromethane, the organic phase was collected, washed and dried, and the organic solvent was removed under reduced pressure to obtain 247 mg of crude product as a white solid, with a yield of 80%. Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0132] 1 H NMR (400MHz, CDCl 3 )δ7.10(d, J=7.9Hz, 1H), 6.33(d, J=7.9Hz, 1H), 4.63(s, 2H), 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com