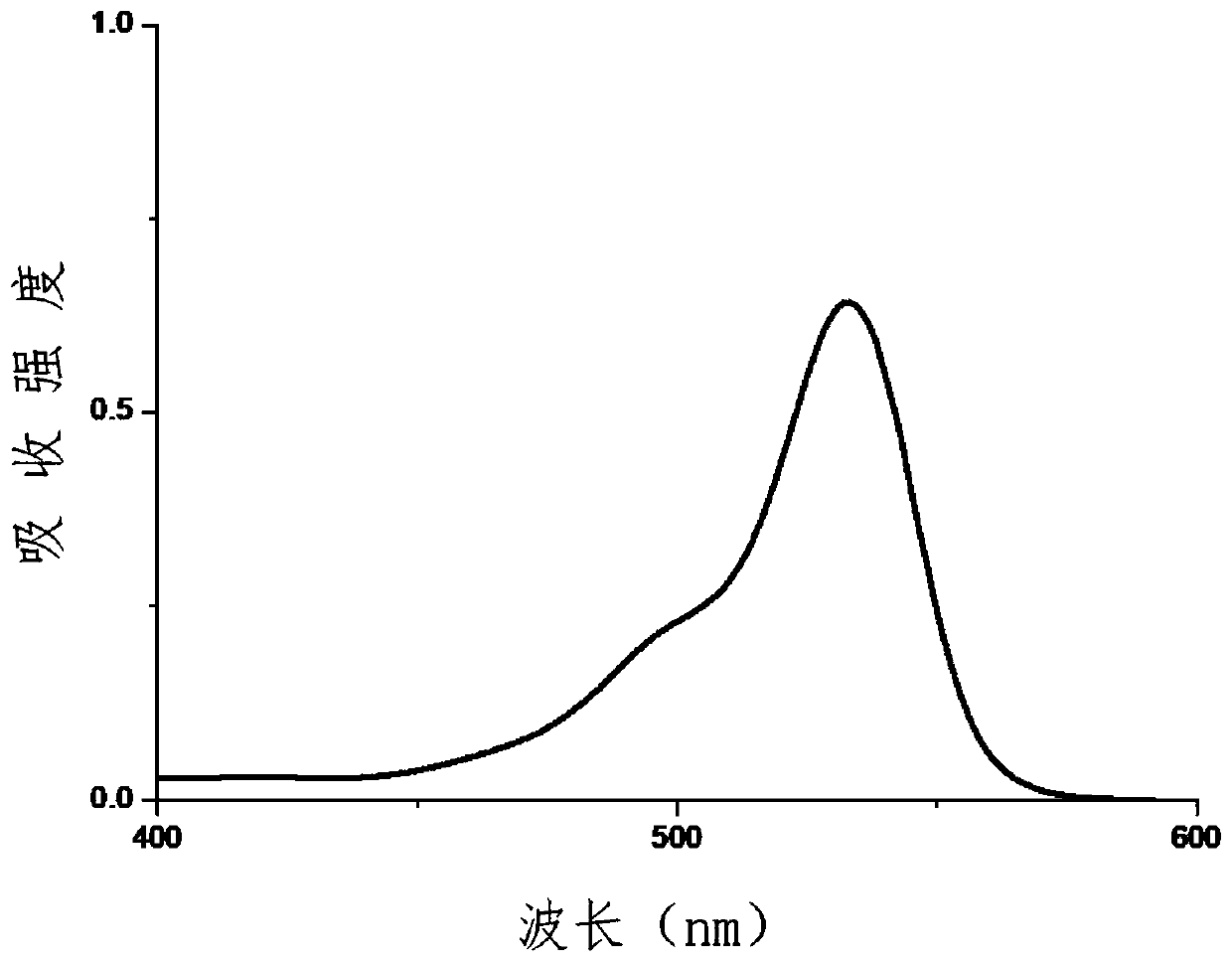
532nm excited rhodamine fluorescent dye and preparation method thereof
A fluorescent dye and fluorescent quantum yield technology, applied in the field of rhodamine-based fluorescent dyes and their preparation, can solve the problems of difficult synthesis, complex structure, rare species and the like, and achieve the effects of high reaction yield and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
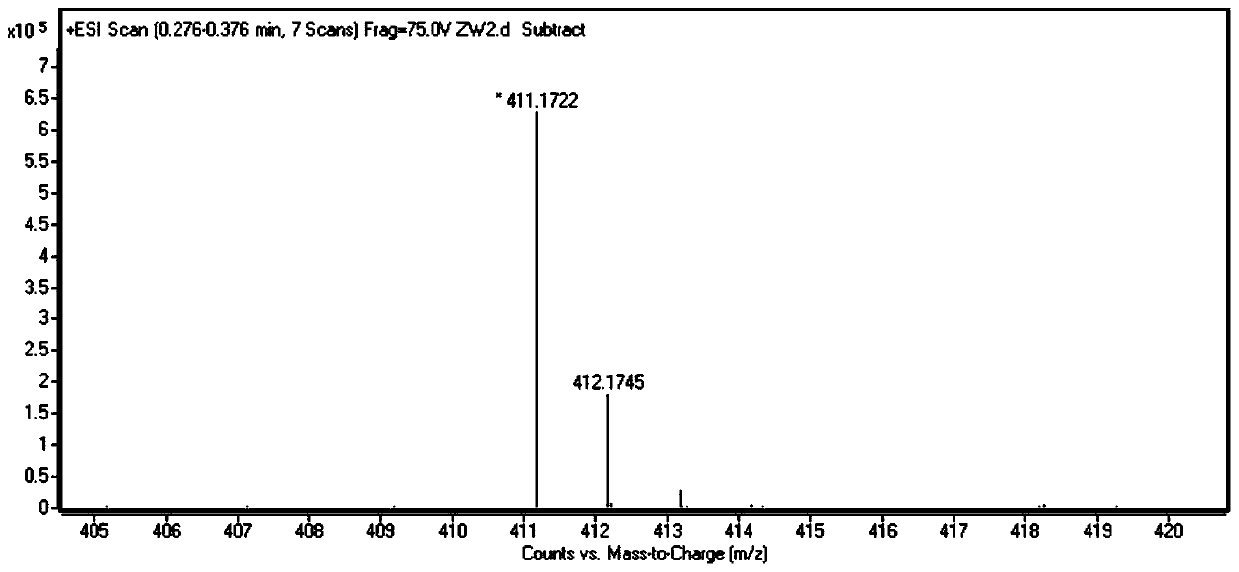
[0037] Synthesis of target dye Rho-1
[0038] Synthesis of Intermediate 4
[0039]
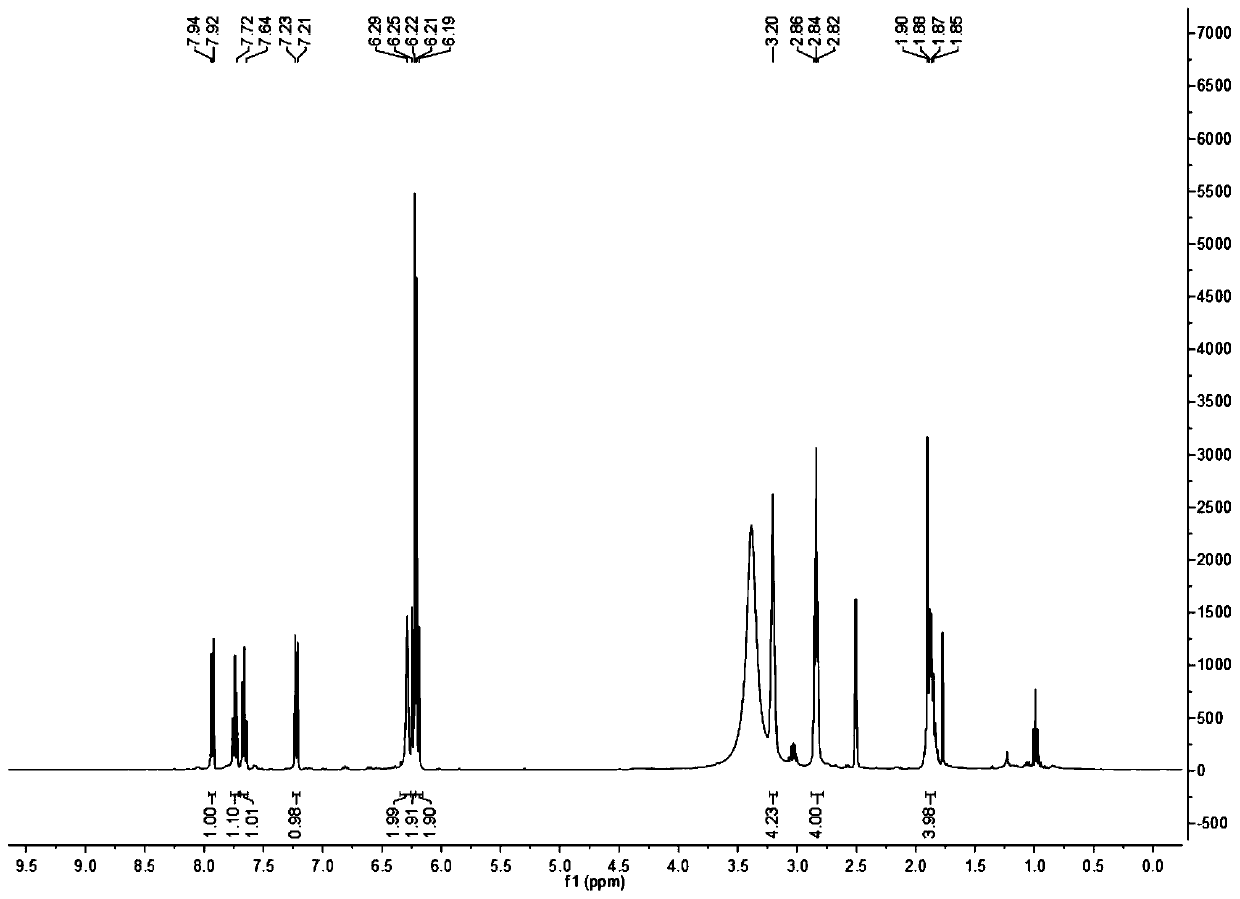
[0040] Dissolve 1 g of 5-hydroxyquinoline in 30 mL of dry tetrahydrofuran solution, add 1.7 g of nickel perchlorate hexahydrate, and add 1 g of sodium borohydride to the reaction system in small portions while stirring at room temperature. After stirring overnight at room temperature, add 5mL deionized water and stir for 10min, pour into 100mL: 200mL of dichloromethane and water mixture, extract and separate, collect the organic phase, dry with anhydrous sodium sulfate, filter, and remove the solvent from the filtrate under reduced pressure 840 mg of solid was obtained, and the yield was 82%. The hydrogen spectrum data of the NMR spectrum are as follows:
[0041] 1 H NMR(400MHz, CDCl 3 )δ6.83(t,J=8.0Hz,1H), 6.13(d,J=1.5Hz,1H), 6.11(d,J=1.6Hz,1H), 4.62(s,1H), 3.99(s, 1H), 3.26(dd,J=7.1,3.8Hz,2H), 2.65(t,J=6.6Hz,2H), 1.97(dd,J=6.6,5.5,4.4Hz,2H).
[0042] Synthesis of Intermediate 5
[0043]
[0044] W...
Embodiment 2
[0056] Synthesis of target dye Rho-2
[0057] Synthesis of Intermediate 6
[0058]
[0059] Dissolve 1 g of 5-hydroxyquinoline in 30 mL of dry tetrahydrofuran solution, add 1.7 g of nickel perchlorate hexahydrate, and add 1 g of sodium borohydride to the reaction system in small portions while stirring at room temperature. After stirring overnight at room temperature, add 5mL deionized water and stir for 10min, pour into 100mL: 200mL of dichloromethane and water mixture, extract and separate, collect the organic phase, dry with anhydrous sodium sulfate, filter, and remove the solvent from the filtrate under reduced pressure 871 mg of solid was obtained, and the yield was 85%.
[0060] 1 H NMR(400MHz, CDCl 3 )δ6.81(d,J=8.1Hz,1H), 6.12(dd,J=8.1,2.5Hz, 1H), 6.01(d,J=2.4Hz,1H), 3.46(s,1H), 3.29( t,J=5.7,2H),2.70(t,J=6.4Hz, 2H),1.93(td,J=11.4,6.3Hz,2H)
[0061] Synthesis of target dye Rho-2
[0062]
[0063] Weigh 100 mg of 5-hydroxytetrahydroquinoline and 215 mg of Intermediate 5, and ad...
Embodiment 3
[0069] Synthesis of target dye Rho-3
[0070] Synthesis of Intermediate 7
[0071]
[0072] Take 1 g of 4-hydroxytetrahydroquinoline in 15 mL of acetic acid, slowly add 1 g of sodium borohydride in a small amount, and after stirring for 30 minutes, slowly add the acetaldehyde solution. After stirring for 2h at room temperature, the reaction was stopped. Remove most of the acetic acid under reduced pressure, quench with sodium carbonate aqueous solution, adjust the pH to neutral, extract with ethyl acetate, wash and dry, remove most of the solvent, and separate on silica gel column. The eluent is petroleum ether: ethyl acetate =50:1 (volume ratio) to obtain 1.09 g of orange solid, with a yield of 93%. The hydrogen spectrum data of the NMR spectrum are as follows:
[0073] 1 H NMR(400MHz, CDCl 3 )δ6.82(d,J=8.0Hz,1H), 6.24(s,1H), 6.12(d,J=8.0Hz,1H), 3.33(q,J=7.1Hz,2H), 3.28(t, J = 5.7Hz, 2H), 2.70 (t, J = 6.4Hz, 2H), 2.02-1.91 (m, 2H), 1.18 (t, J = 7.1Hz, 3H).
[0074] Synthesis of I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com