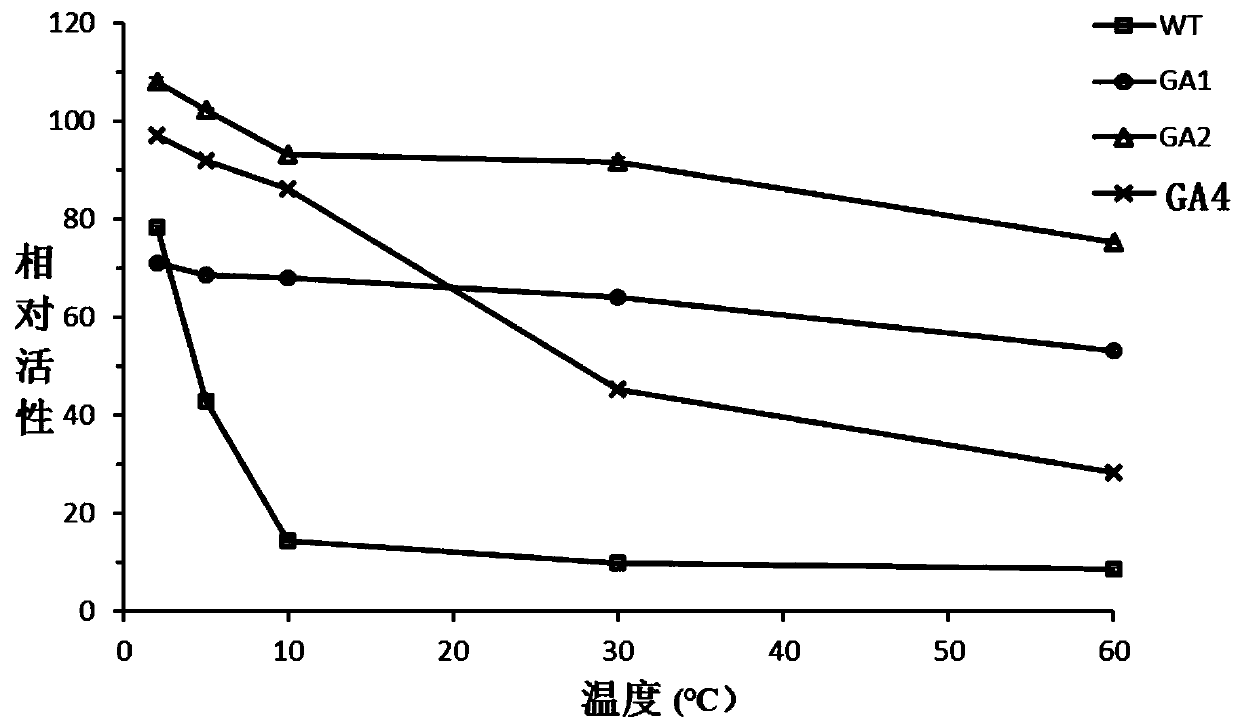
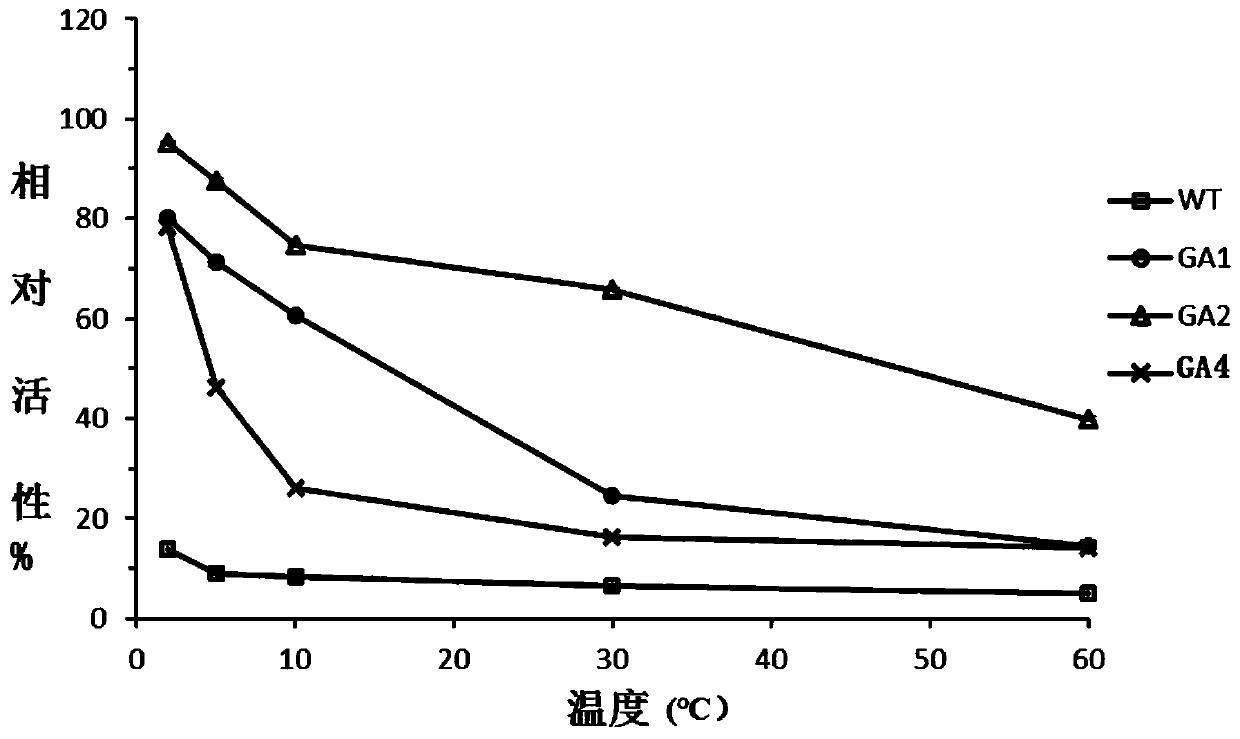
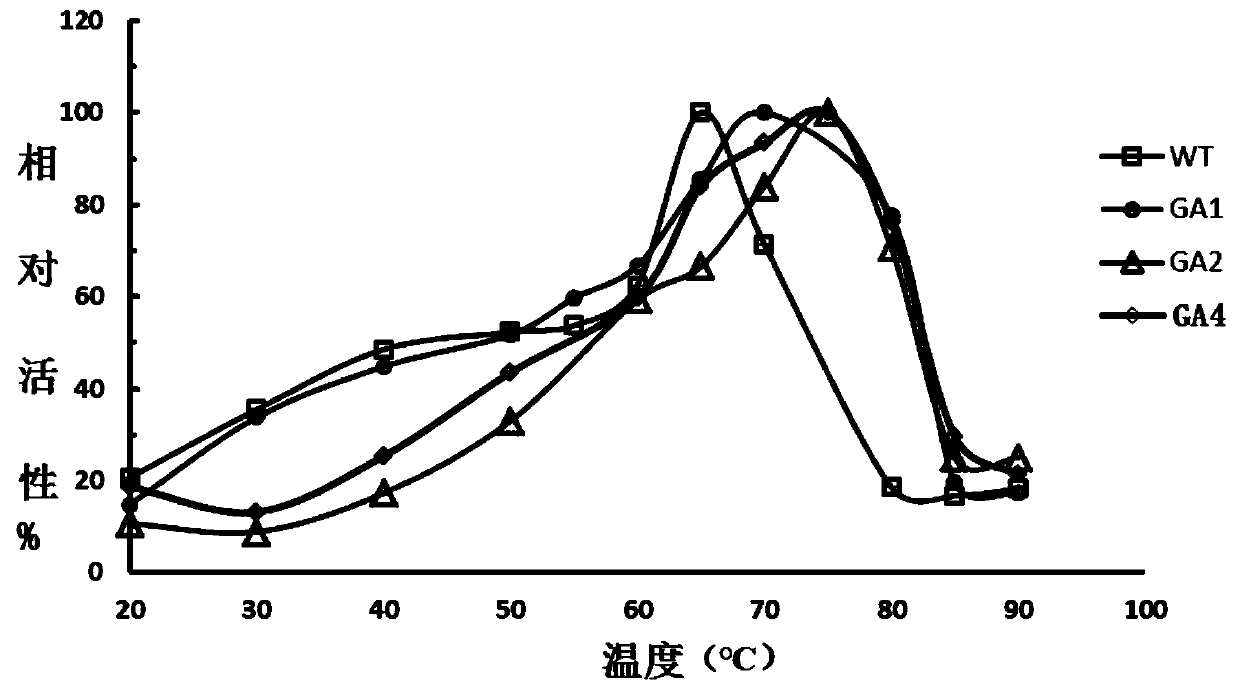
Glucoamylase mutants GA1, GA2 and GA4 with improved specific activity and thermal stability, and genes and applications of glucoamylase mutants
A glucoamylase and thermal stability technology, applied in the field of genetic engineering, can solve the problems of consumption of starch substrate, low starch utilization rate and ethanol yield, etc., and achieves improved specific activity and thermal stability, good thermal stability, The effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The site-directed mutation of embodiment 1 glucoamylase
[0055] Using the plasmid pPIC9-Tlga1931 sequence of glucoamylase TlGA1931 as a template, the 132nd base of the amino acid sequence of glucoamylase TlGA1931 was mutated from Ser to Cys, the 492nd base was mutated from Tyr to Cys, and the 548th base The glucoamylase mutant GA1 is obtained by mutating Leu to Cys, and the 562nd base is mutated from Ala to Cys, and the 108th base of the amino acid sequence of the mutant GA1 of glucoamylase TlGA1931 is mutated from Gln to Glu to obtain glucose starch For the enzyme mutant GA2, the 475th base of the amino acid sequence of the mutant GA2 of the glucoamylase T1GA1931 was mutated from Ser to Ala to obtain the glucoamylase mutant GA4. The primers for each round of site-directed mutagenesis are listed in the table below:
[0056] Table 1 Primers required for this experiment
[0057]
Embodiment 2
[0058] The construction of embodiment 2 glucoamylase engineering strains
[0059] (1) Construction of expression vector and expression in yeast
[0060] Using the glucoamylase recombinant plasmid pPIC9-Tlga1931 as a template, the mutants were amplified using site-directed mutagenesis reagents. After verification by nucleic acid gel, add 1 μL of DMT enzyme to the PCR product, mix well and incubate at 37°C for 1 hour. Take 2-5 μL of the PCR product digested with DMT enzyme and transform it into DMT competent cells by heat shock. Positive transformants were picked for DNA sequencing, and the transformants with the correct sequence were used to prepare a large number of recombinant plasmids. Linearize expression plasmid vector DNA with restriction endonuclease Bgl II, transform yeast GS115 competent cells by electroporation, culture at 30°C for 2-3 days, pick transformants grown on MD plates for further expression experiments, specific operations Please refer to the Pichia expr...
Embodiment 3
[0061] The preparation of embodiment 3 recombinant glucoamylases
[0062] (1) Massive expression of glucoamylase at shake flask level in Pichia pastoris
[0063] Screen the transformant with higher enzyme activity, inoculate it in a 1L Erlenmeyer flask with 300mL of BMGY liquid medium, culture it on a shaker at 30°C and 220rpm for 48h; centrifuge at 4500rpm for 5min, discard the supernatant, and then add 200mL containing 0.5% Methanol BMMY liquid medium, 30°C, 220rpm induction culture for 48h. During the induction culture period, methanol solution was added once every 24 hours to compensate for the loss of methanol, so that the methanol concentration was kept at about 0.5%; centrifuged at 12,000×g for 10 minutes, the supernatant fermentation liquid was collected, and the enzyme activity was detected and analyzed by SDS-PAGE protein electrophoresis.
[0064] (2) Purification of recombinant glucoamylase
[0065] Collect the recombinant glucoamylase supernatant from the shake f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com