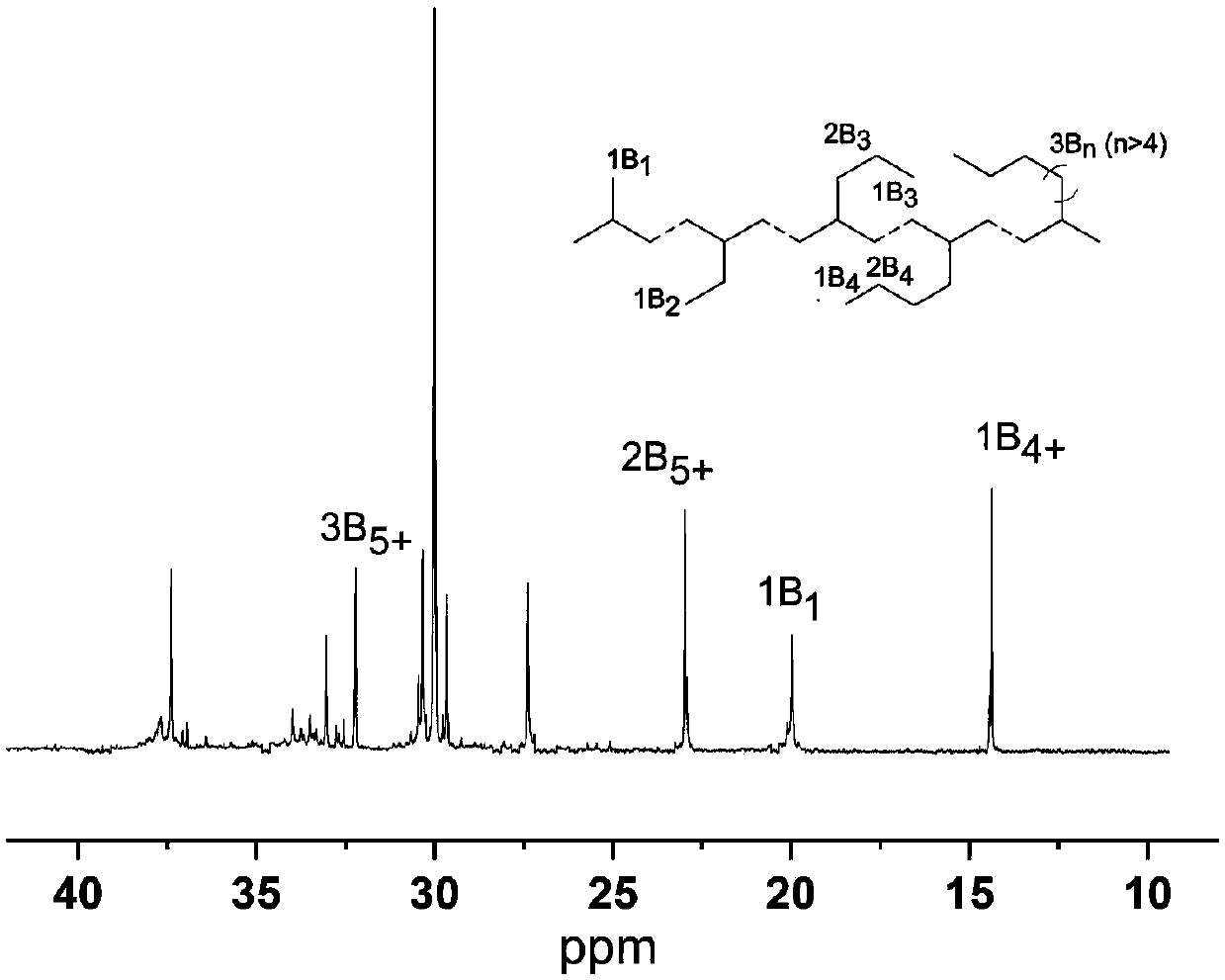
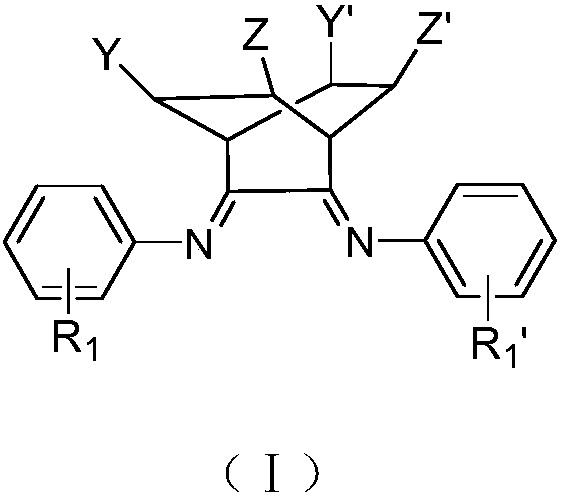
Alpha-diimine ligand compound, complex and preparation method of polyolefin lubricating oil base oil
A technology of ligand compounds and diimine, which is applied in the preparation of imino compounds, nickel organic compounds, chemical instruments and methods, etc., can solve the problems of inability to prepare lubricating base oil, high content of short-chain branches, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The synthesis of embodiment 1 ligand L1
[0117] Synthesis of bicyclo[2,2,2]octane-2,3-dione:
[0118]
[0119] In a pressure bottle, add 8.0 g of 1,3-cyclohexadiene (0.1 mol) and 43 g of vinylene carbonate (0.5 mol), and react at 180° C. for 24 hours. After cooling to room temperature, methanol precipitated to obtain the addition product. The above product was dissolved in tetrahydrofuran, added with 50 mg of Pd / C, and reacted at 60° C. for 12 h under hydrogen atmosphere. After filtration, the solvent was removed by rotary evaporation. The above product was dissolved in 5 g of potassium hydroxide in ethanol solution, refluxed for 8 h, washed with water and dried to obtain diol. Dissolve the above product in a mixture of 200mL of dichloromethane and 8mL of dimethyl sulfoxide, add 12mL of trifluoroacetic anhydride dropwise at -78°C, add 25mL of triethylamine dropwise after reacting for 2 hours, and continue to stir for 2 hours. Dry over sodium sulfate and recrystal...
Embodiment 2
[0121] The synthesis of embodiment 2 ligand L2
[0122]According to the synthesis method of ligand L1 in Example 1, 3-(2-thienyl)aniline was used instead of 2-(2-furyl)aniline, and other operating conditions were the same, and the yield was 80.3%. 1 H NMR (400MHz, CDCl 3 , ppm): 7.79, 7.72, 7.51, 6.94 (m, 8H, Ph-H), 7.69, 7.40, 7.17 (m, 6H, thienyl), 2.56 (m, 2H, CH), 1.71 (m, 4H, CH 2 ), 1.45 (m,4H,CH 2 ).
Embodiment 3
[0123] Synthesis of Example 3 Ligand L3
[0124] According to the synthesis method of ligand L1 in Example 1, 4-(2-pyridyl)aniline was used instead of 2-(2-furyl)aniline, and other operating conditions were the same, and the yield was 72.9%. 1 H NMR (400MHz, CDCl 3 , ppm): 8.35, 8.21, 7.76, 7.65 (m, 8H, Ph-H), 8.50, 7.51, 7.26, 7.00 (m, 8H, pyridyl), 2.48 (m, 2H, CH), 1.84 (m, 4H ,CH 2 ), 1.53 (m,4H,CH 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pour point | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com