Catalyst for synthesizing diphenyl carbonate, preparation method thereof and process
A diphenyl carbonate and catalyst technology, applied in the field of catalytic synthesis of diphenyl carbonate, can solve the problems of deactivation of catalyst active components, low activity or selectivity, and difficulty in repeated use, and achieve high catalytic activity and high catalytic performance Activity, energy barrier lowering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Prepare 1mol / L lithium nitrate solution, impregnate 20g ZSM-5 molecular sieves in 1L lithium nitrate solution, stir at room temperature for 6 hours, filter solid-liquid separation, dry the solid at 120°C for 12 hours, and then bake at 550°C for 3 hours . The above steps were repeated 2 more times. Molecular sieve M1 was prepared.
[0052] Molecular sieve M1 was vacuum dried at 120° C. for 4 hours. Prepare a 0.8mol / L tin chloride solution, and impregnate the molecular sieve M1 with equal volume. Dry at room temperature for 12 hours. Molecular sieve M1 was then further vacuum dried at 120°C for 4 hours.
[0053] Prepare a 0.8mol / L zirconium nitrate solution, and impregnate the molecular sieve M1 with equal volume. Dry at room temperature for 12 hours. Precursor P1 is obtained.
[0054] Then the precursor P1 was placed in 1L saturated ammonia water and soaked at room temperature for 4 hours. Dry at room temperature for 12 hours.
[0055] The dried solid was calcin...
Embodiment 2-17
[0058] Change the type of lithium salt, solution concentration, solid-to-liquid ratio, treatment time, drying temperature, drying time, and treatment times, and the rest of the conditions are the same as in Example 1. Obtain LiZSM-5 molecular sieve. As shown in Table 1.
[0059] Table 1.
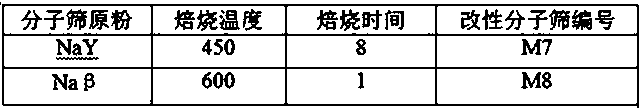
[0060]
[0061] The type of molecular sieve, the calcination temperature and time were changed, and other conditions were the same as in Example 1 to obtain LiY molecular sieve and Liβ molecular sieve.
[0062] Table 2.
[0063]
[0064] Use the lithium type molecular sieve in table 1 and table 2, change the metal salt solution type, concentration, solid-liquid ratio, drying condition, treatment times of active component, all the other conditions are identical with embodiment 1, obtain catalyst precursor P, as table 3.
[0065] table 3.
[0066]
[0067] Use the catalyst precursor in Table 3, change the soaking time of saturated ammonia water, drying condition, treatment times ...
Embodiment 18
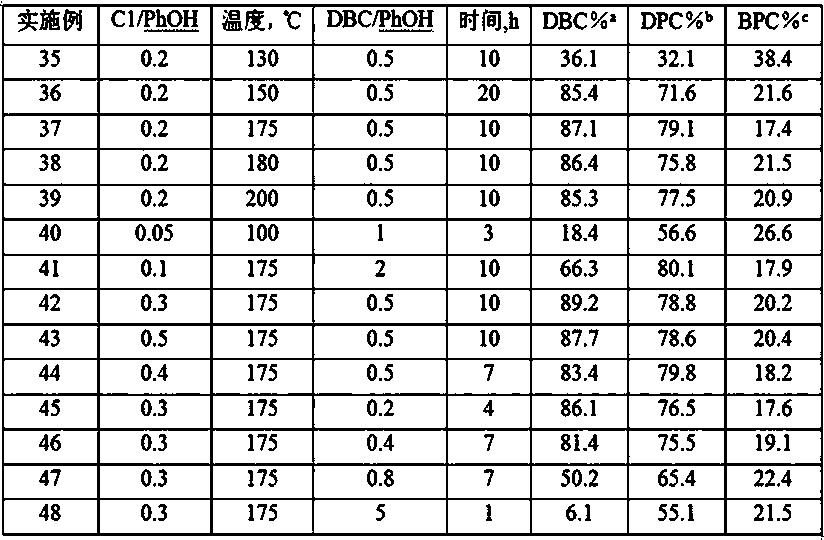
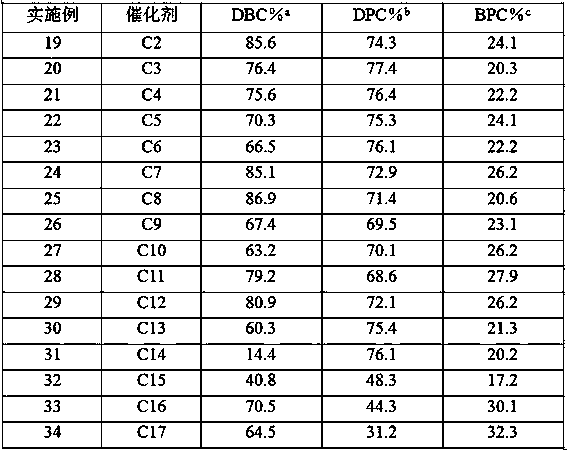
[0071] Weigh 10g of the catalyst C1 prepared in Example 1 above and place it in a 300mL stainless steel reactor, replace the air in the reactor with N2, then fill it with 50.0g of phenol and 64.3g of dibenzyl carbonate, raise the temperature to 175°C, and react for 10 Cool after 1 hour, reaction product is carried out chromatographic analysis, in molar basis, the conversion rate that obtains dibenzyl carbonate (DBC) is 86.3%, the selectivity of diphenyl carbonate (DPC) is 76.5%, benzyl phenyl carbonate (BPC) selectivity was 21.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com