Chloroquine spray and preparation method thereof
A technology of chloroquine and spray, which is applied in the direction of pharmaceutical formulations, antiviral agents, local antibacterial agents, etc., can solve the problems affecting the quality and curative effect of drugs, the low bioavailability of tablets, and the difficulty of dividing doses or coatings, etc., to achieve Achieve long-term protection, promote absorption and residence time, and have less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of chloroquine phosphate spray
[0056] The chloroquine phosphate spray is composed of powder A and solution B; the powder A contains chloroquine phosphate, carrageenan and a sweetener, and the solution B is a potassium chloride aqueous solution containing borneol and menthol, and the pH value is adjusted to 6.0.
[0057] In terms of mass volume percentage, the dosage of each component is: 8% carrageenan, 1% sweetener, 0.8%-0.9% potassium chloride.
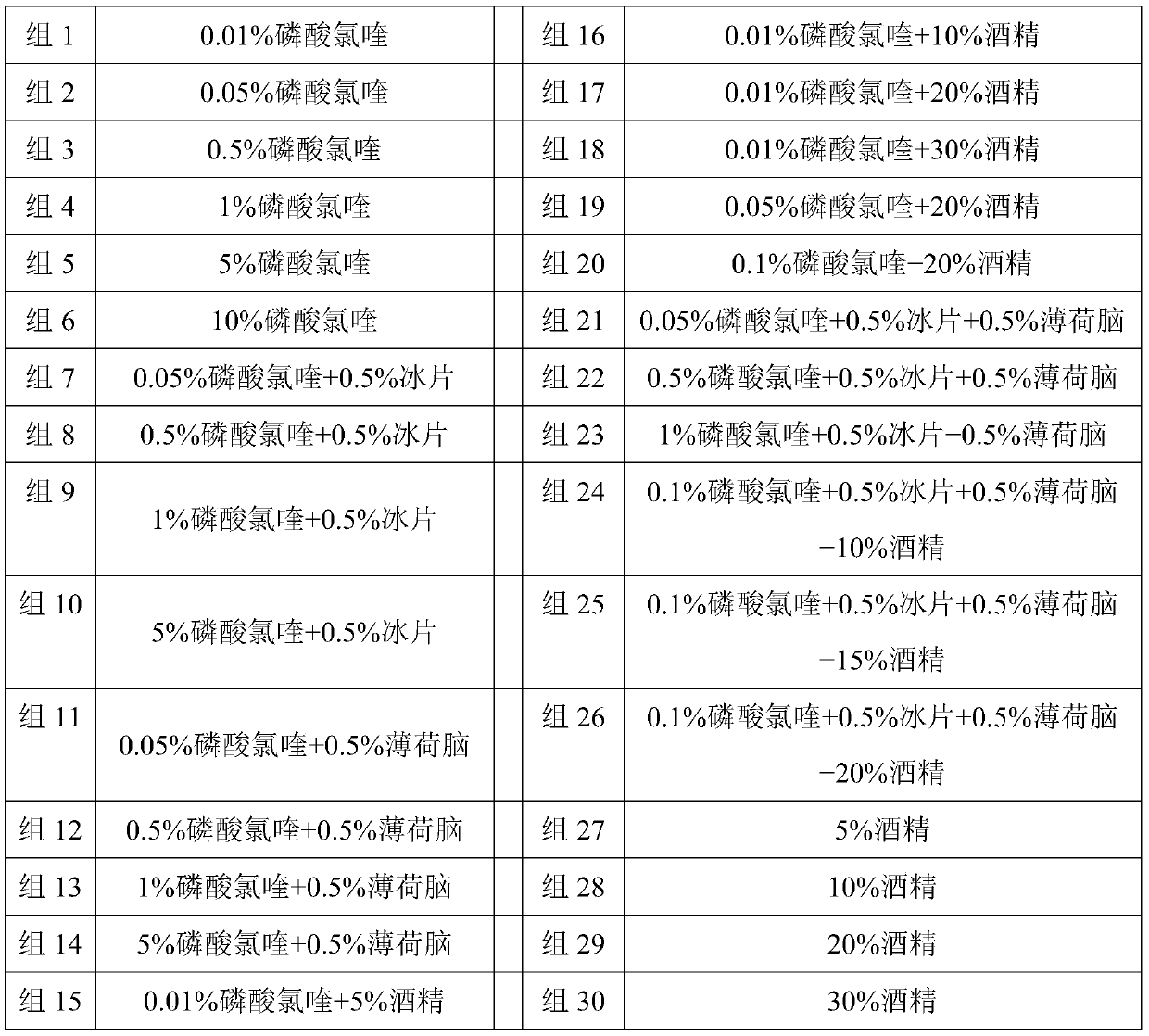
[0058] The amounts of chloroquine phosphate, borneol, menthol, and ethanol are shown in Table 1. A series of chloroquine phosphate sprays were prepared according to different proportions of chloroquine phosphate, borneol, menthol, and ethanol (alcohol).
[0059] The preparation method of powder A of the spray is: formulating carrageenan into an aqueous solution with a mass concentration of 10%-20%, adding sweetener, heating to 30-100°C, adding chloroquine phosphate, cooling, drying, and powdering to obtain pow...
Embodiment 2
[0064] Example 2 Bacteriostatic test of chloroquine phosphate spray
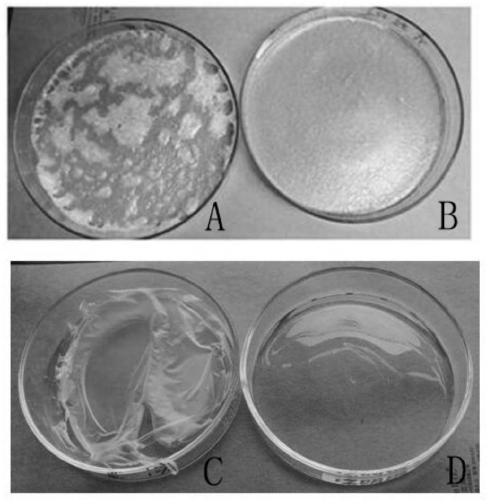
[0065] 1. Test the antibacterial effect of each group of chloroquine phosphate sprays prepared in Example 1.
[0066] The antibacterial test method is as follows: take the nutrient agar medium (Sabao's agar medium) of the 3rd to 14th generation of the strain and wash the lawn with 5mL 0.03mol / L phosphate buffer solution (hereinafter referred to as PBS) to make the bacteria Mix the solution uniformly, use a standard bacterial turbidity tube, dilute with the above PBS to a bacterial concentration of 5.0×l0 5 ~4.5×10 6 / mL of bacterial suspension.
[0067] Take 5 mL of the sample solution (sample 1:1 dilution) and 4 tubes of the control sample solution (sample without antibacterial components), add 100 μL of the above bacterial suspension respectively, mix well, and start timing for 2 minutes. Mix thoroughly and dilute appropriately. Take 0.5 mL of each dilution and place it on the culture medium of the corresponding...
Embodiment 3
[0081] Example 3 Quality test of chloroquine phosphate spray
[0082] The sprays shown in Table 5 were prepared with reference to Example 1, and the sprays of different formulations were tested in a double-blind manner, and the irritation, bitterness, stickiness of spraying into the oral cavity, and acceptability were scored after the trial. The statistical results of trial use of its products are shown in Table 5.
[0083] Table 5 Quality test of chloroquine phosphate spray
[0084]
[0085]
[0086] In this trial, the score was irritation from 1 to 10 points, the higher the score, the stronger the irritation; the bitterness from 1 to 10 points, the higher the score, the stronger the bitterness; the viscosity from 1 to 10 points, the more the score High viscosity is stronger; acceptability ranges from 1 to 10 points, the higher the score, the stronger the acceptability. It can be seen from the results in Table 5 that the chloroquine phosphate spray of the present invention is mild...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com