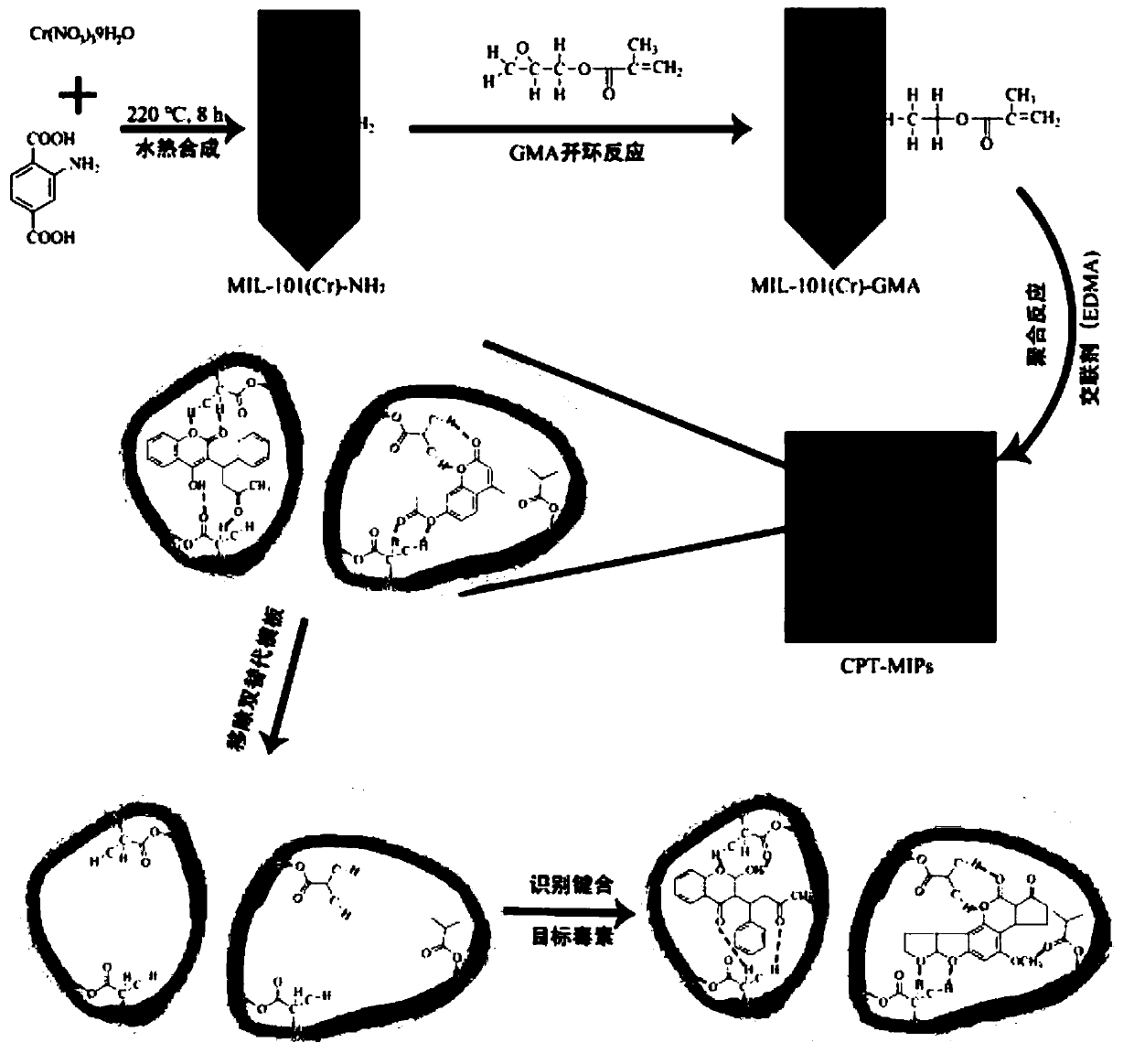
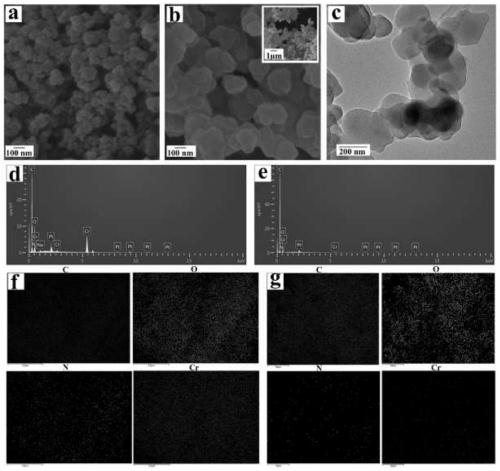
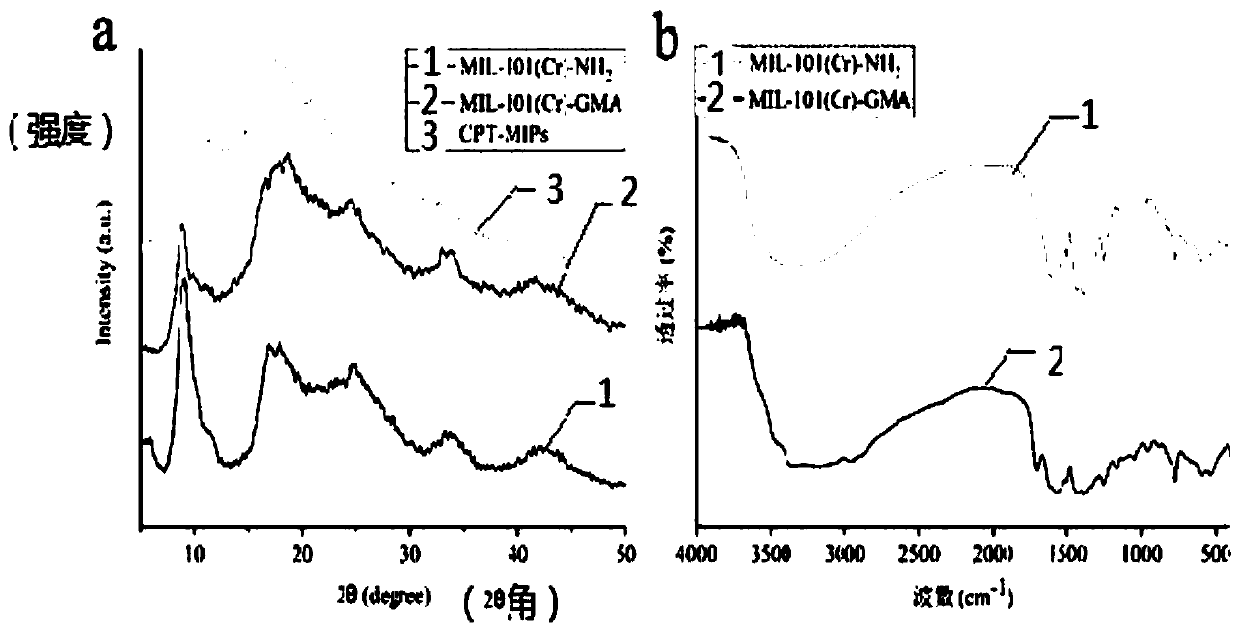
Dual-template molecule amino functionalized metal organic framework imprinted polymer as well as synthesis method and application thereof
A metal-organic framework and imprinted polymer technology, which is applied in the field of analytical chemistry, can solve the problems of not being able to adsorb multiple types of mycotoxins at the same time, and achieve the effects of rapid adsorption equilibrium, cost reduction, and stable and reliable methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1CPT-MIPs
[0036] 1.1 Experimental reagents
[0037] Aminoterephthalic acid (NH 2 -H 2 BDC) (98%, McLean); aflatoxins (AFB1, AFB2, AFG1, AFG2) (98%, Aladdin); warfarin (98%, Adamas Reagent Co. Ltd); 7-acetoxy - 4-Methylcoumarin (98%, Adamas); glycidyl methacrylate (GMA) (AR, McLean).
[0038] 1.2 Preparation of CPT-MIPs
[0039] (1) MIL-101(Cr)-NH 2 Synthesis
[0040] Add 3.2g (10mmol) Cr(NO 3 )9H 2 O, 1.5g (8.28mmol) NH 2 -H 2 BDC and 60mL deionized water were stirred at room temperature for 1 hour, and then the jacket of the stainless steel reactor was tightened. Using the hydrothermal synthesis method, the oven is controlled by a program to control the temperature, within 30 minutes, the temperature is raised from 50°C to 150°C, and kept at 150°C for 12h. After cooling to room temperature, the product was separated by centrifugation, and the unreacted NH 2 -H 2 BDC. The product was dried overnight in a vacuum oven at 100 °...
Embodiment 2
[0053] Example 2 CPT-MIPs adsorption performance experiment
[0054] 2.1 Preparation of double substituted template standard solution
[0055] Warfarin and 7-acetoxy-4-methylcoumarin concentrations were 0.5, 1.0, 2.0, 3.0, 5.0, 10, 15, 20, 30, 40 and 50 μg mL -1 standard solution, using methanol: water (1:9, V / V) preparation, ready to use.
[0056] 2.2 Static adsorption experiment
[0057]Weigh 10 mg of the eluted and dried CPT-MIPs and mix them with 5 mL of the above-mentioned double substitution template standard solutions of different concentrations in a 10 mL centrifuge tube, shake at 90 rpm for 1 h with an oscillator, and filter the supernatant with a filter membrane. Determine the concentration of the double template in the solution, and calculate the adsorption amount of CPT-MIPs to warfarin sodium and 7-acetoxy-4-methylcoumarin according to formula 3.1:
[0058] Q=(C 0 -C t )*V / m (3.1)
[0059] in,
[0060] Q is the adsorption capacity of CPT-MIPs, (mg g -1 ); ...
Embodiment 3
[0087] Application in embodiment 3 actual samples
[0088] 3.1 Actual sample pretreatment
[0089] Rice samples were purchased from local supermarkets (Henan, Zhengzhou), crushed, and passed through a 40-mesh sieve. Weigh 40g of the above sample, 4gNaCl and 100mL extract (acetonitrile:water=9:1) into a 250mL Erlenmeyer flask and shake for 1h. After filtering with glass fiber filter paper, dilute 20 mL with distilled water 4 times, filter the mixed solution until clear, collect and set aside.
[0090] 3.2 Preparation of CPT-MIPs solid phase extraction column
[0091] Weigh 200 mg of CPT-MIPs after elution and drying, transfer to the SPE small column with the lower sieve plate, add 5 mL of methanol dropwise to wet it, use a capillary glass tube to stir out the air bubbles in the adsorbent, place the upper sieve Plate and ensure that the adsorbent in the column does not leak, ready for use.
[0092] 3.3 Optimization of SPE process of CPT-MIPs column
[0093] (1) Selection ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com