Penicillin G acylase mutant and application thereof in enzymatic synthesis of cefamandole
A mutant, penicillin technology, applied in biochemical equipment and methods, enzymes, enzymes, etc., can solve the problems of not meeting the needs of actual industrial production, low synthetic hydrolysis, and only conversion rate, and achieve broad industrial application prospects, hydrolysis The effect of reducing the activity and increasing the ratio of synthesis to hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example illustrates the site-directed mutagenesis of penicillin G acylase.
[0034] Carry out single-point mutation, two-point combined mutation and three-point combined mutation at the following three sites of penicillin G acylase: the 186th arginine (R) is mutated into another amino acid residue selected from the following C amino acid (A), aspartic acid (D), or lysine (L); the 187th phenylalanine (F) is mutated to any other amino acid residue (isoleucine (I), Valine (V)); the 329th phenylalanine (F) mutation is another amino acid residue selected from the following alanine (A), glycine (G), isoleucine (I) , Valine (V), Serine (S). Use oligo7 software to design blunt-end primers, and the gene sequence (SEQ ID NO: 1) encoded by the wild-type penicillin G acylase amino acid sequence (SEQ ID NO.2) derived from Achromobacter xylosoxidans PX02 is a template, and then Perform PCR site-directed mutagenesis.
[0035] The base sequence of the primer is:
[0036]
...
Embodiment 2
[0049] This example illustrates the induced expression and purification of penicillin G acylase mutants.
[0050] 1. Induced expression of penicillin G acylase mutant
[0051] (1) The recombinant transformants obtained in Example 1 were inoculated into LB medium containing kanamycin (peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, pH 6.5, shake culture at 37°C for 12h, Obtain the seed solution.
[0052] (2) Insert the inoculum amount of 2% (v / v) into a 250mL Erlenmeyer flask equipped with 40mL LB medium, place it on a shaker at 37°C and 180rpm for culture, when the culture solution OD 600 When it reaches 0.6, add IPTG with a final concentration of 0.1mmol / L as an inducer, and induce at 16°C for 24h.
[0053] (3) Centrifuge the culture medium, collect the cells, and wash twice with saline to obtain resting cells. Suspend the resulting resting cells in a pH 8.0 buffer, ultrasonically break in an ice bath, and centrifuge to collect the supernatant, which is the crude enzyme solu...
Embodiment 3
[0057] This experiment illustrates the application of penicillin G acylase mutants in the synthesis of cefamandole.
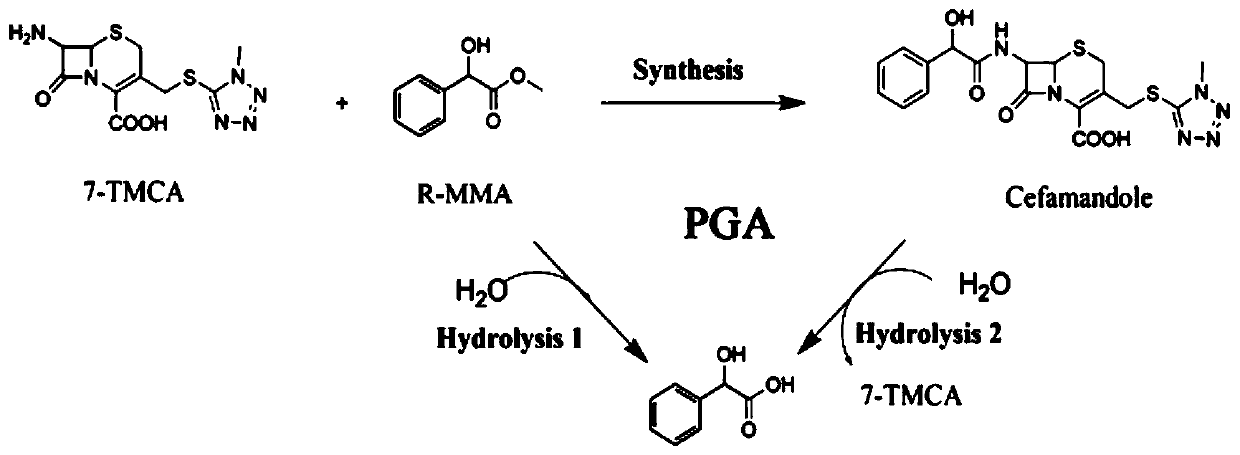
[0058] The technical scheme adopted in this embodiment is as follows figure 1 shown.
[0059] Depend on figure 1 The reaction formula shows that penicillin G acylase can not only condense the mother nucleus 7-TMCA and the side chain methyl mandelate to produce the product cefamandole, but also decompose the product to form the mother nucleus and mandelic acid, and at the same time, the methyl mandelate itself can also be hydrolyzed into mandelic acid.
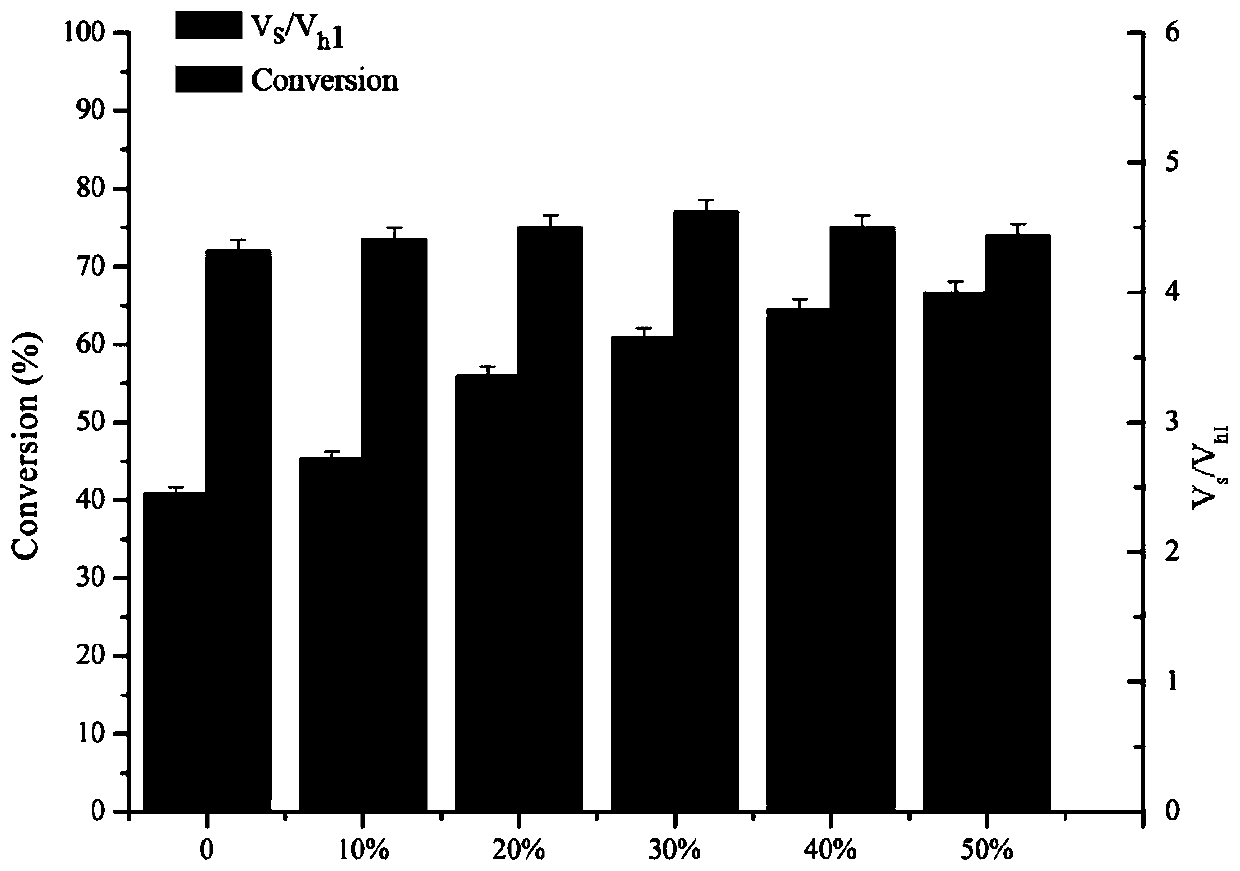
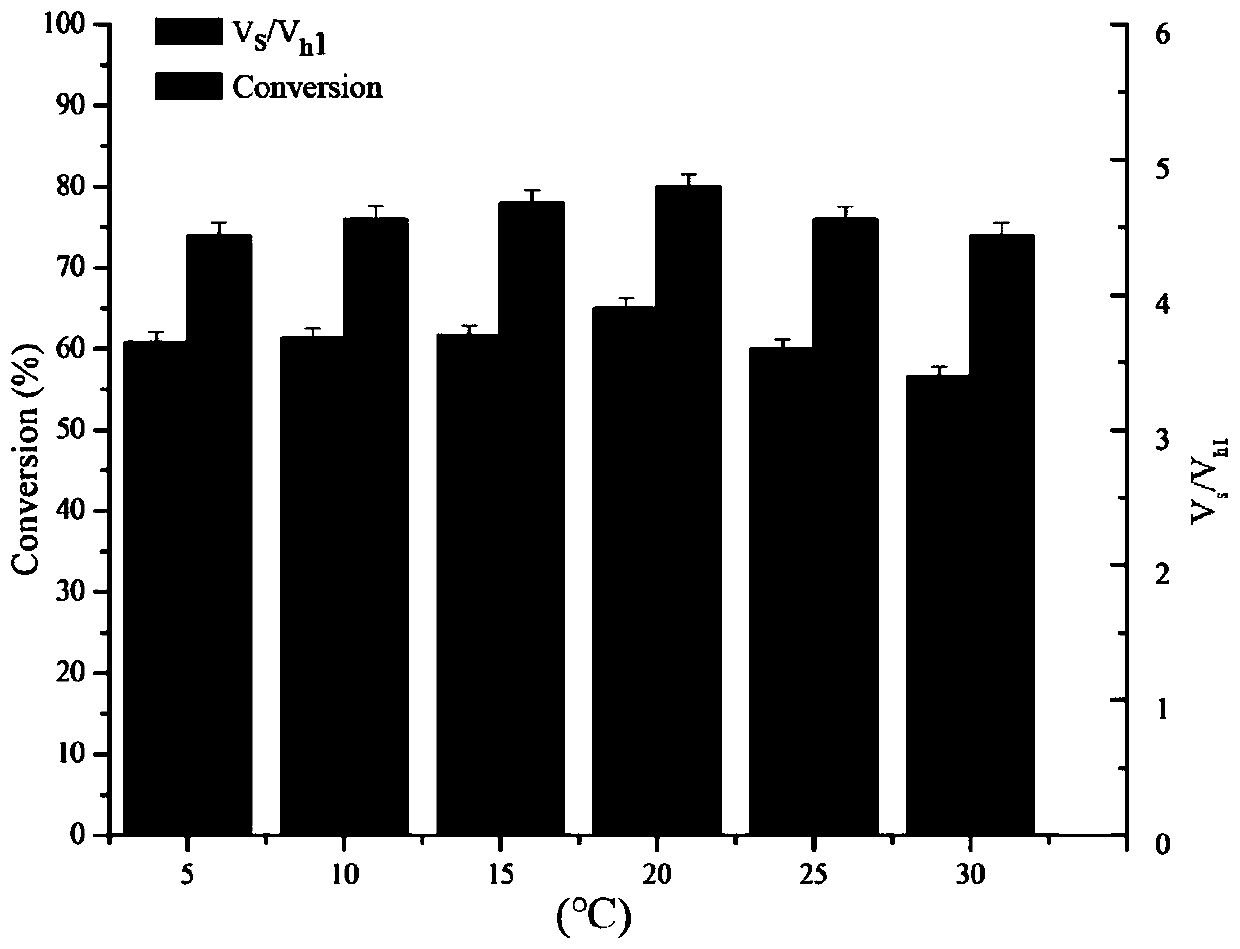
[0060] Reaction system: use pH 6.5 phosphate buffer as the reaction medium. The total reaction volume is 2mL, which contains 50mM 7-TMCA, 150mM R-MMA (R-methyl mandelate), add penicillin acylase mutant enzyme solution (prepared in Example 1-2), and the reaction temperature is 25 ° C, The reaction was carried out for 5 hours at a rotational speed of 200 rpm, and samples were taken regularly. The sample was d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Extend | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com