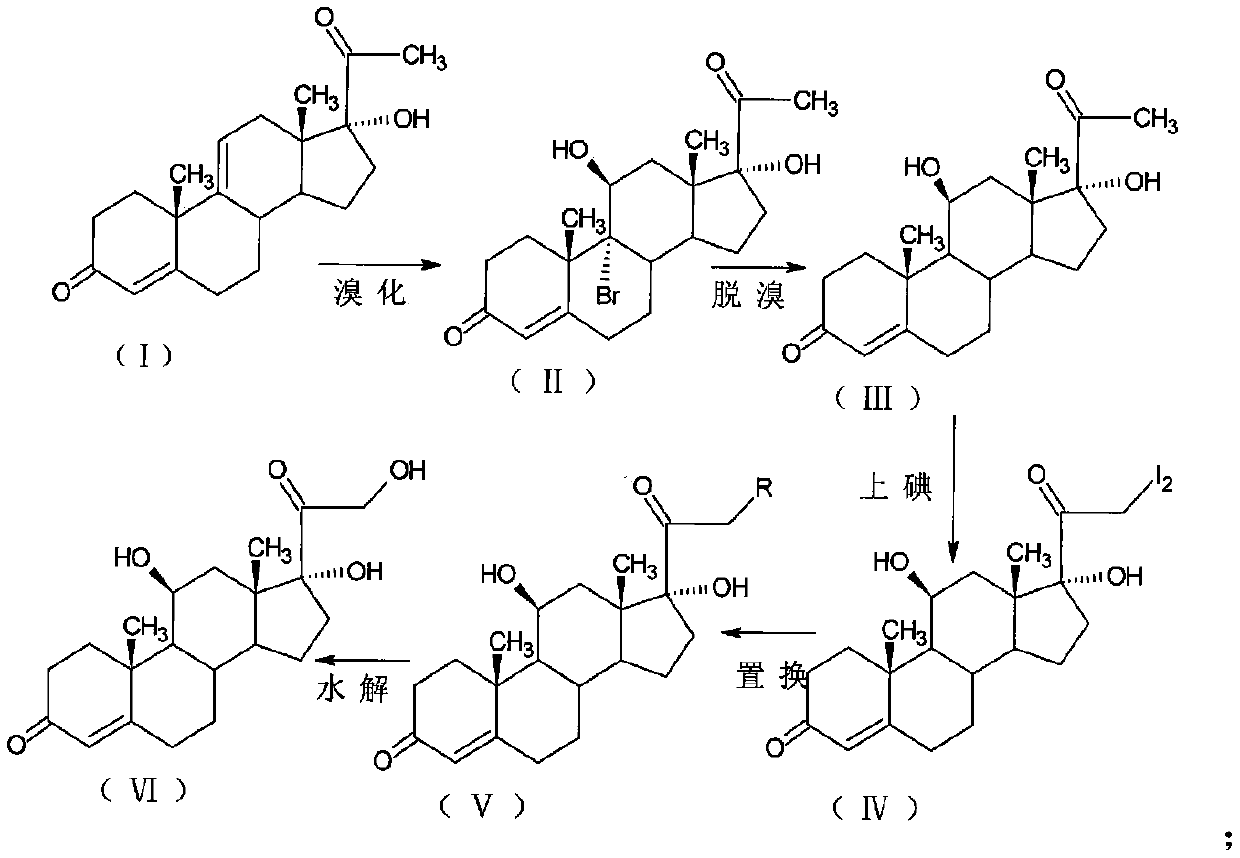
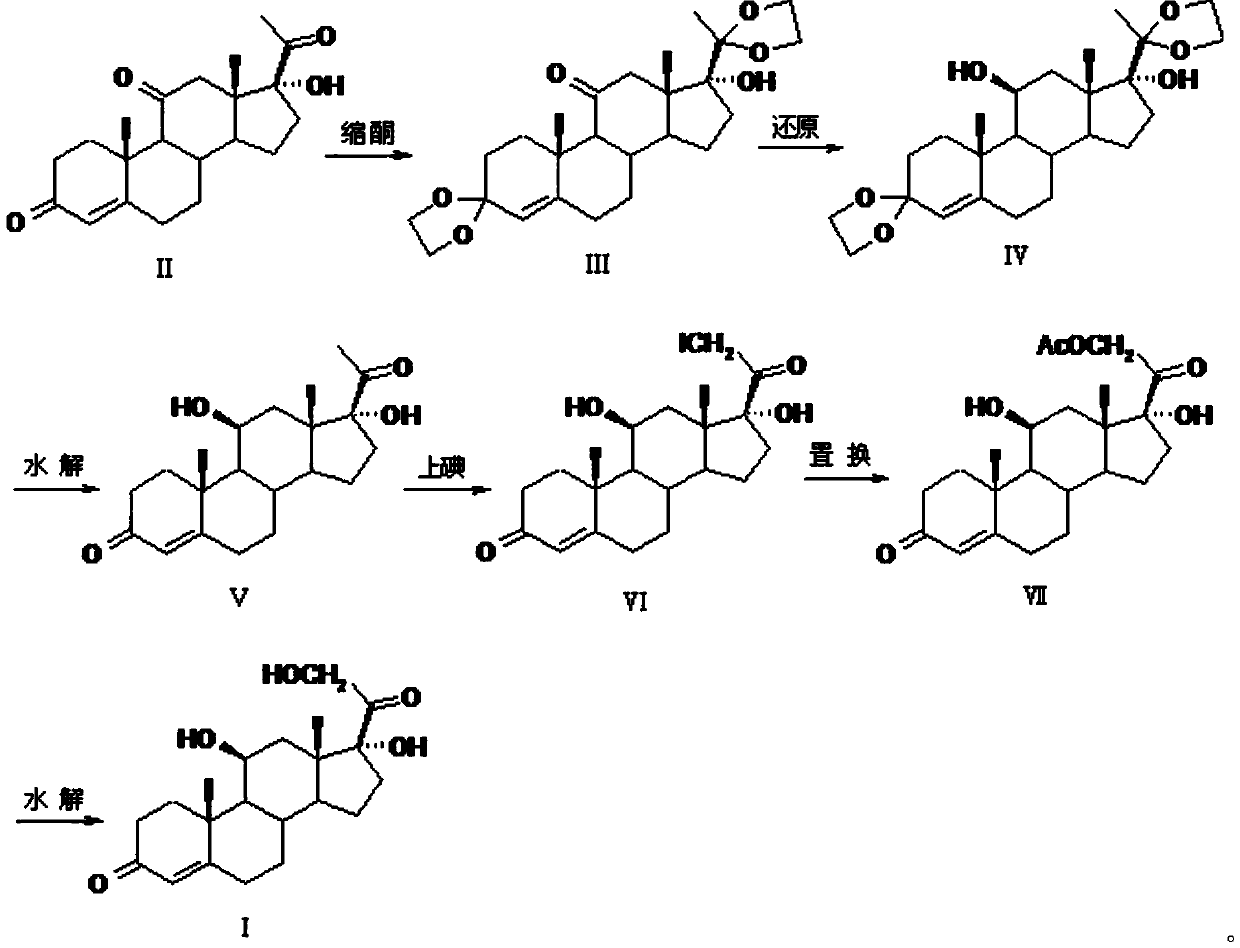
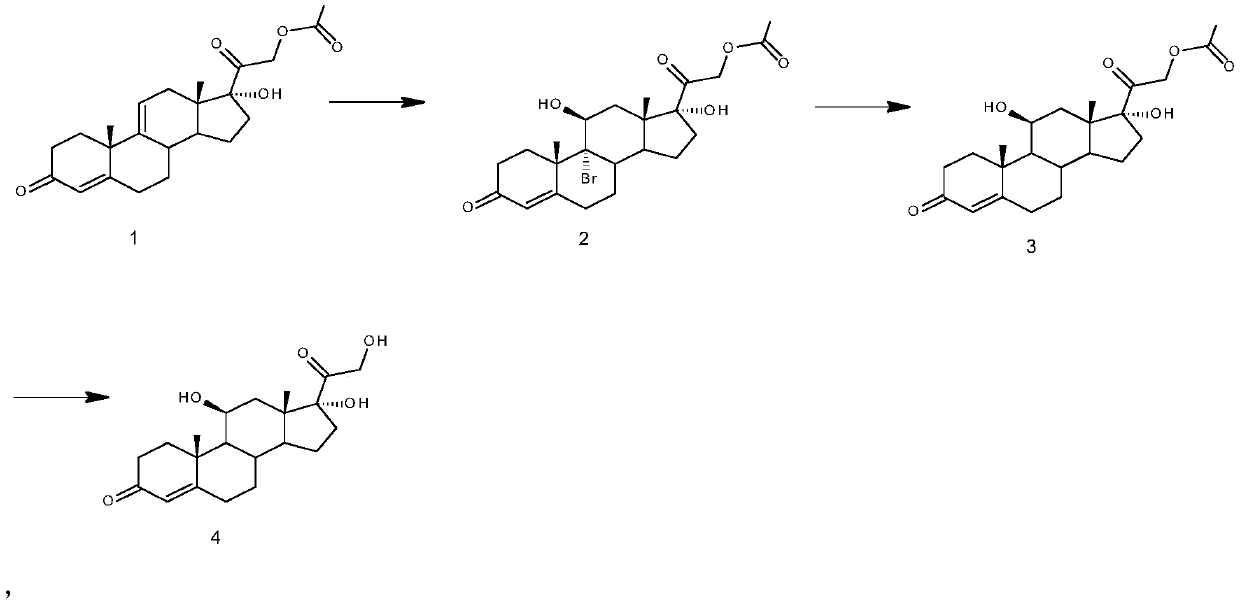
Preparation method of high-purity hydrocortisone
A technology of hydrocortisone and hydrocortisone acetate, which is applied in the direction of organic chemistry and steroids, can solve the problems of unfriendly environment, low yield, high toxicity, etc., and achieve low requirements for reaction devices and improve product quality. The effect of purity and good market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 A kind of preparation method of high-purity hydrocortisone comprises the steps:
[0027] 1) Bromohydrin reaction: 50 grams of 17α-hydroxypregna-4,9(11)-diene-3,20-dione-21-acetate (1) was added to 300 ml of acetone, and the mass concentration was 5 % perchloric acid aqueous solution 70 milliliters, be cooled to 5 ℃, add N-bromosuccinimide 25 grams, stir reaction, after reaction is finished, neutralize with the sodium sulfite aqueous solution of 5% mass concentration, concentrate organic solvent, water Analyze, filter, obtain 66 grams of intermediate (2).
[0028] 2) Debromination reaction: under nitrogen protection, 66 grams of intermediate (2) were dissolved in 130 milliliters of N,N-dimethylformamide and 300 milliliters of tetrahydrofuran, and 33 milliliters of thioglycolic acid, 40 grams of zinc powder and 2 grams of hexahydrate were added Chromium trichloride, control temperature 5 ℃ of stirring reactions, after reaction is finished, neutralize with th...
Embodiment 2
[0030] Embodiment 2 A kind of preparation method of high-purity hydrocortisone comprises the steps:
[0031] 1) Bromohydrin reaction: 50 grams of 17α-hydroxypregna-4,9(11)-diene-3,20-dione-21-acetate (1) was added to 1500 milliliters of acetone at a mass concentration of 10 50 milliliters of fluoroboric acid aqueous solution, be cooled to-5 ℃, add 60 grams of dibromodimethylhydantoin and stir reaction, after reaction is finished, be neutralized with the sodium carbonate aqueous solution that mass concentration is 10%, condense organic solvent, water analysis , and filtered to obtain 67 g of intermediate (2).
[0032]2) Debromination reaction: under nitrogen protection, 67 grams of intermediate (2) was dissolved in 300 milliliters of dimethyl sulfoxide and 500 milliliters of 2-methyltetrahydrofuran, and 14 milliliters of thioglycolic acid, 15 grams of zinc powder and 3 grams of hexahydrate were added Chromium trichloride, control the temperature-5 ℃ of stirring reaction, after...
Embodiment 3
[0034] Embodiment 3 A kind of preparation method of high-purity hydrocortisone comprises the steps:
[0035] 1) Bromo-hydroxyl reaction: 50 grams of 17α-hydroxypregna-4,9(11)-diene-3,20-dione-21-acetate (1) was added to 1000 milliliters of methyl ethyl ketone, and the mass concentration was 25 milliliters of 30% methanesulfonic acid aqueous solution is cooled to 0 DEG C, add 45 grams of dibromodimethylhydantoin, stir and react, after the reaction is completed, neutralize with ammonia water of 30% mass concentration, concentrate the organic solvent, water analysis, After filtration, 65 g obtained Intermediate 2.
[0036] 2) Debromination reaction: under nitrogen protection, 65 grams of intermediate (2) was dissolved in 200 milliliters of N,N-dimethylformamide and 750 milliliters of acetone, and 70 milliliters of thioglycolic acid, 65 grams of zinc powder and 6.5 grams of hexahydrate were added Chromium trichloride, 10 ℃ of stirring reactions of control temperature, after the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com