Magnesium-containing raw material recycling treatment method
A processing method and a resource-recycling technology, which are applied in the field of resource recovery of magnesium in ores and smelting waste residues, and can solve the problems of heavy metal discharge, unrecoverable magnesium, and waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
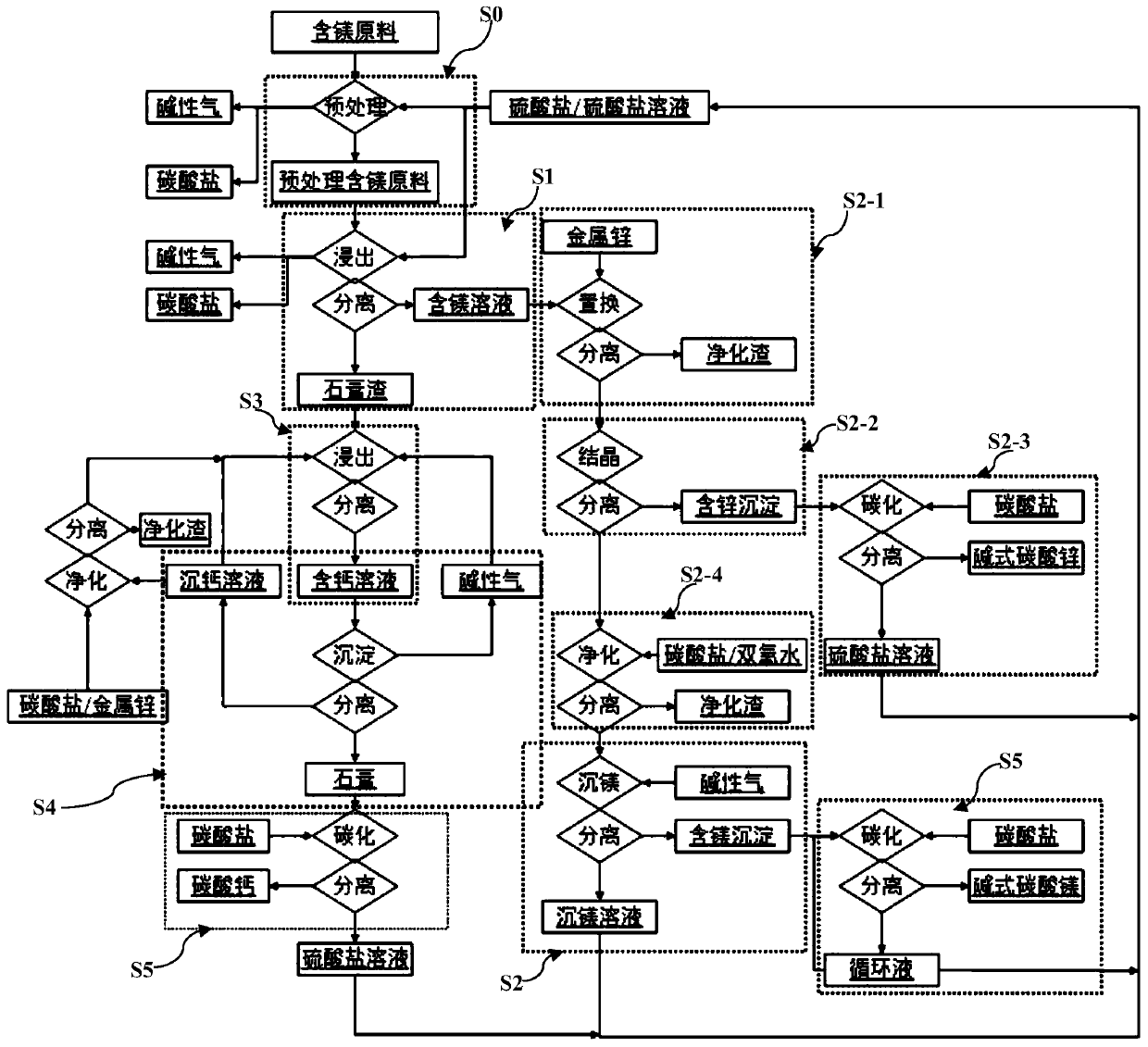
[0095] (1) Add 1kg of monoethanolamine, 0.25kg of threonine, and 0.2kg of phenylalanine to a 10L reaction kettle, set the volume to about 8.5L, add 1kg of dolomite (containing 12.7% of magnesium and 21.8% of calcium), and heat to 60°C, use sulfuric acid to adjust the pH to less than 4.0, react for 2 hours, filter, and wash with 1L of pure water to obtain 9L of filtrate and gypsum residue;
[0096] (2) transfer the obtained filtrate of step (1) into another reaction kettle, use sodium hydroxide water to adjust the pH value between 9.0-9.1, add 0.5kg of sodium carbonate, and filter to obtain calcium carbonate precipitation and filtrate;
[0097] (3) step (2) gained filtrate is transferred in another reactor, adds sodium carbonate 1.5kg, uses sodium hydroxide to maintain pH value between 9.7-9.9 during, reacts 5h, filters, obtains filtrate and basic magnesium carbonate precipitation;
[0098] (4) step (1) gained gypsum slag is transferred in another 15L reactor, adds 1kg magnesi...
Embodiment 2
[0102] (1) Mix 100g of magnesium smelting waste slag (4.5% magnesium and 50.1% calcium) with 100g ammonium sulfate solid, put it into a tube furnace, use argon to blow, and use two-stage 200ml water to absorb the tail gas. Pretreatment at ℃ for 10 hours to obtain 170g of pretreated magnesium smelting slag and 200ml of ammonia water (ammonia content 11.5%);
[0103] (2) Prepare 1L of 0.1mol / L magnesium sulfate and 0.5mol / L ammonium sulfate solution, add the pretreated magnesium smelting slag obtained in step (1) into the solution, heat to 100°C, react for 0.5h, and filter to obtain 0.95L filtrate and gypsum residue;
[0104] (3) Transfer the filtrate obtained in step (2) into another beaker, add sodium hydroxide to adjust the pH value to 11.1-11.2, maintain the reaction temperature at 95°C, and filter for 2 hours to obtain magnesium hydroxide precipitate and sulfate filtrate .
[0105] (4) The magnesium hydroxide precipitate obtained in step (3) is transferred to a beaker, 10...
Embodiment 3
[0109] (1) Add 55.6g of ammonium sulfate and 20g of magnesium sulfate to a 1L beaker, set the volume to 800ml, add 400g of dirty acid neutralization slag (containing 10% zinc, 5.5% magnesium, and 10% calcium), start stirring, and heat to 120 ℃, react for 2 hours, control the reaction pressure to 0.1Mpa, and filter to obtain 700ml of filtrate and gypsum slag;
[0110] (2) Add 10g of zinc powder to the filtrate gained in step (1), react for 5h, and filter to obtain purified residue and filtrate;
[0111] (3) Add the filtrate obtained in step (2) into another beaker, heat and evaporate to 400ml, cool to 0°C, and filter to obtain ammonium zinc sulfate solid and filtrate;
[0112] (4) Transfer the zinc ammonium sulfate solid obtained in step (3) into another beaker, add 500ml of water, use ammonia water to adjust the pH value between 8.0-8.2, pass through carbon dioxide at 100ml / min for 2 hours, filter, and obtain the basic formula Zinc carbonate products;
[0113] (5) The filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com