Ginsenoside derivative and synthesis method and application thereof
A technology of ginsenoside and synthesis method, which is applied in the field of medicine, can solve the problems of high toxicity of normal cells, insufficient solubility, poor killing effect of tumor cells, etc., and is conducive to large-scale production and purification, improving application value, strong resistance The effect of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A synthetic method for ginsenoside derivatives, comprising the following steps:
[0038] Get 1g ginsenoside (20S)-Rh2 and dissolve in 600mL acetonitrile-EDTA disodium mixed solution (the volume ratio of acetonitrile and EDTA disodium is 1:1, and the concentration of EDTA disodium in the mixed solution is 4×10 -4 mol / L). 4942 mg of potassium peroxymonosulfonate ( mono-persulfate compound) and 2104mg of NaHCO 3 The mixture was slowly added to the (20S)-Rh2 solution prepared above, and the reaction pH was adjusted to 6.0-7.0.
[0039] Weigh 1245 mg of epoxidized diketal catalyst (Shi epoxidation diketal catalyst (Ketone)), completely dissolve in 150 mL of acetonitrile, add dropwise to the above (20S)-Rh2 reaction solution, and stir overnight at room temperature. Reaction solution is filtered, and after decompression reclaims acetonitrile, carry out ODS column chromatography, 50%-90% methanol gradient elution, through TLC detection (thin-layer chromatography) merge iden...
Embodiment 2
[0045] Cytotoxicity test
[0046] 20(S)-Rh2E2 was dissolved in DMSO (dimethyl sulfoxide) to a final concentration of 50 mmol / L, and stored at -40°C before use. Normal human liver cell LO2 and various tumor cells (lung cancer cell A549, lung cancer cell H1299, cervical cancer cell HeLa, liver cancer cell HepG2, mouse lung cancer cell LLC-1 and breast cancer cell MCF-7) were inoculated in a 96-well plate , and then exposed to different concentrations of 20(S)-Rh2E2 solutions or DMSO as a control for 72 hours. Subsequently, 10 μL of MTT reagent was added to each well, and 100 μL of solubilization buffer (10% sodium dodecyl sulfate added to 0.01 mol / L hydrochloric acid) was added after 4 hours to incubate overnight. Measure the absorbance at 570nm with a microplate reader, and calculate the cell viability by the following formula:
[0047] Cell viability (%)=(Atreated–Abackground) / (Acontrol–Abackground)×100.
[0048] Table 1
[0049]
[0050]
[0051] The test results ar...
Embodiment 3
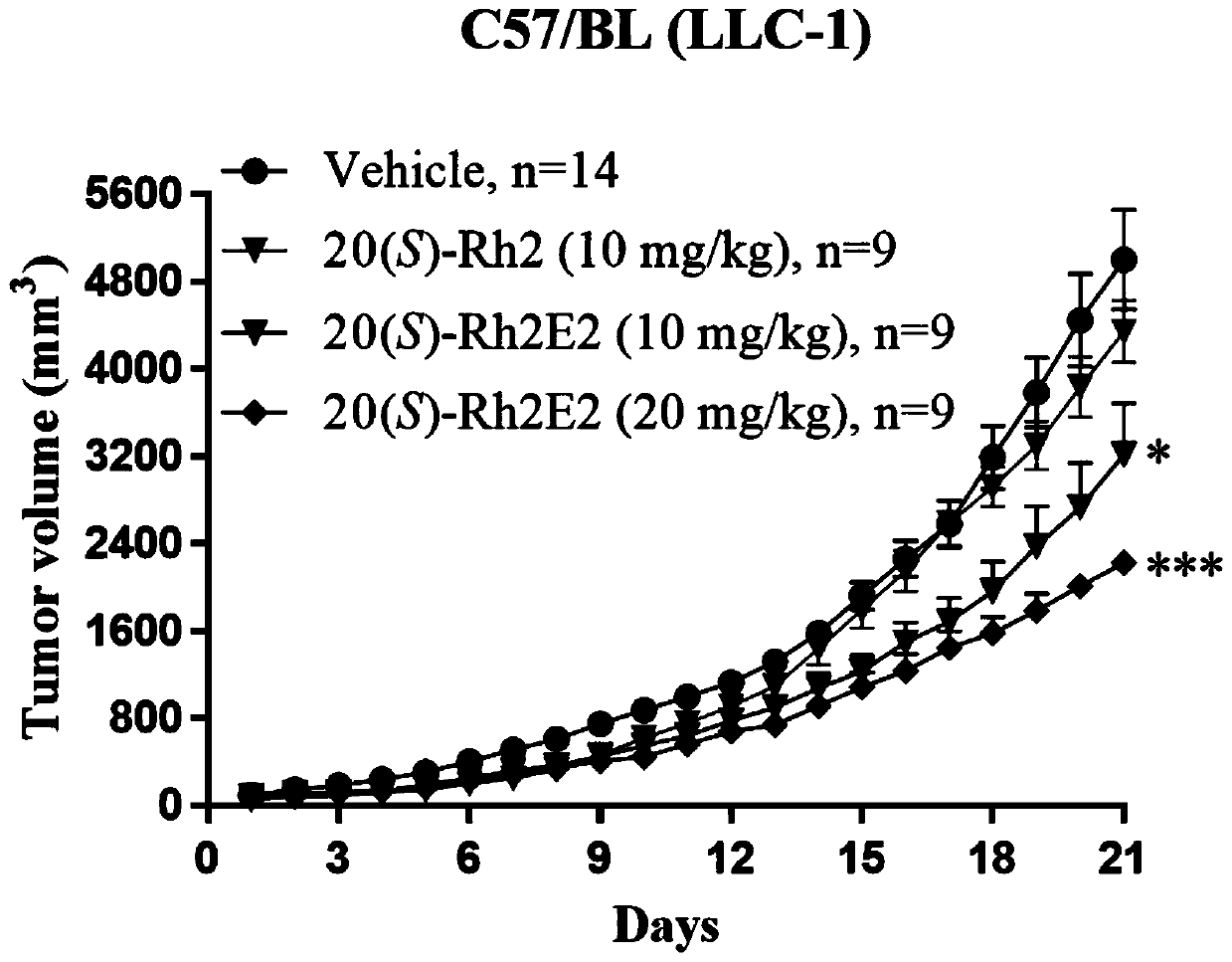
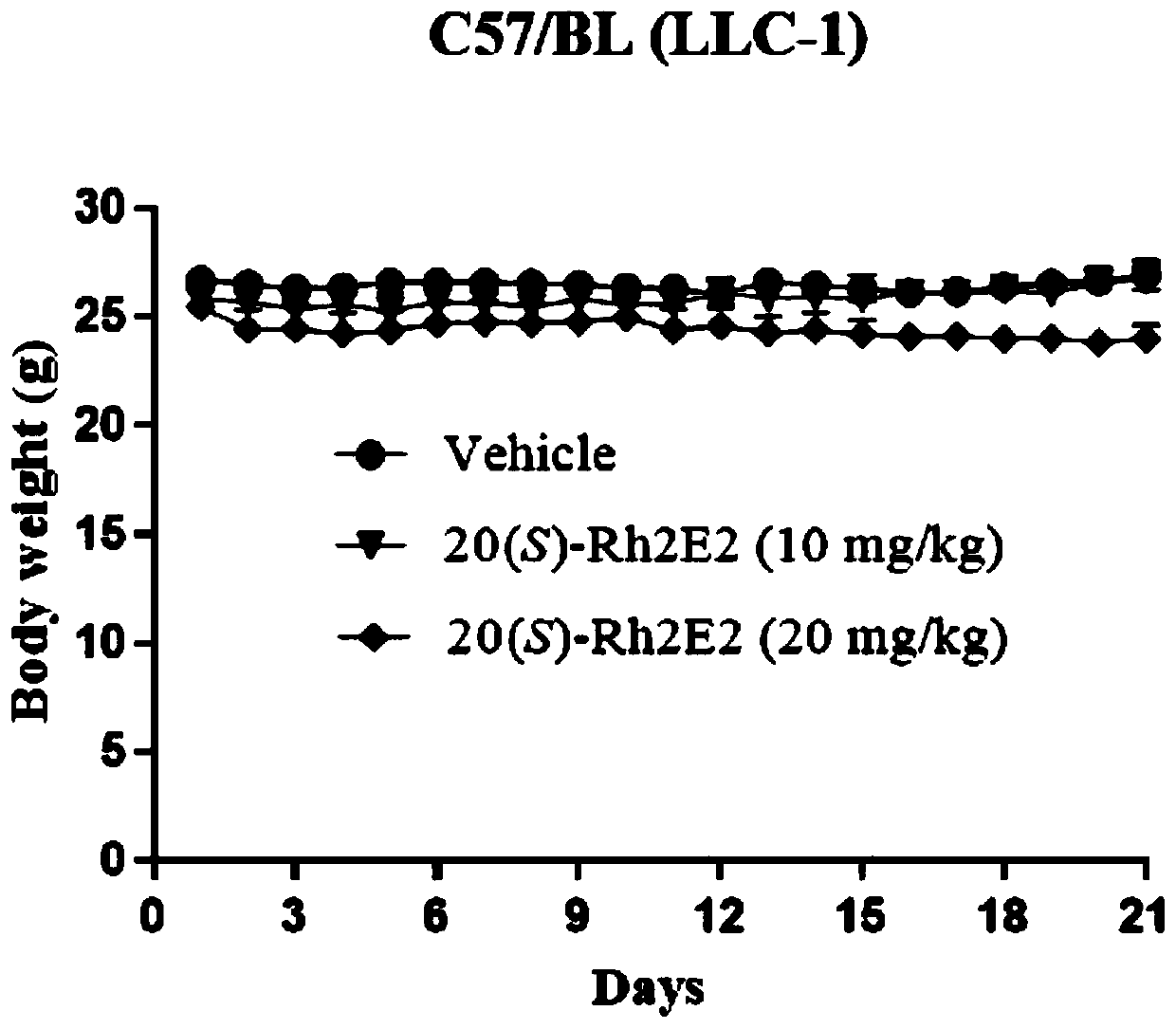
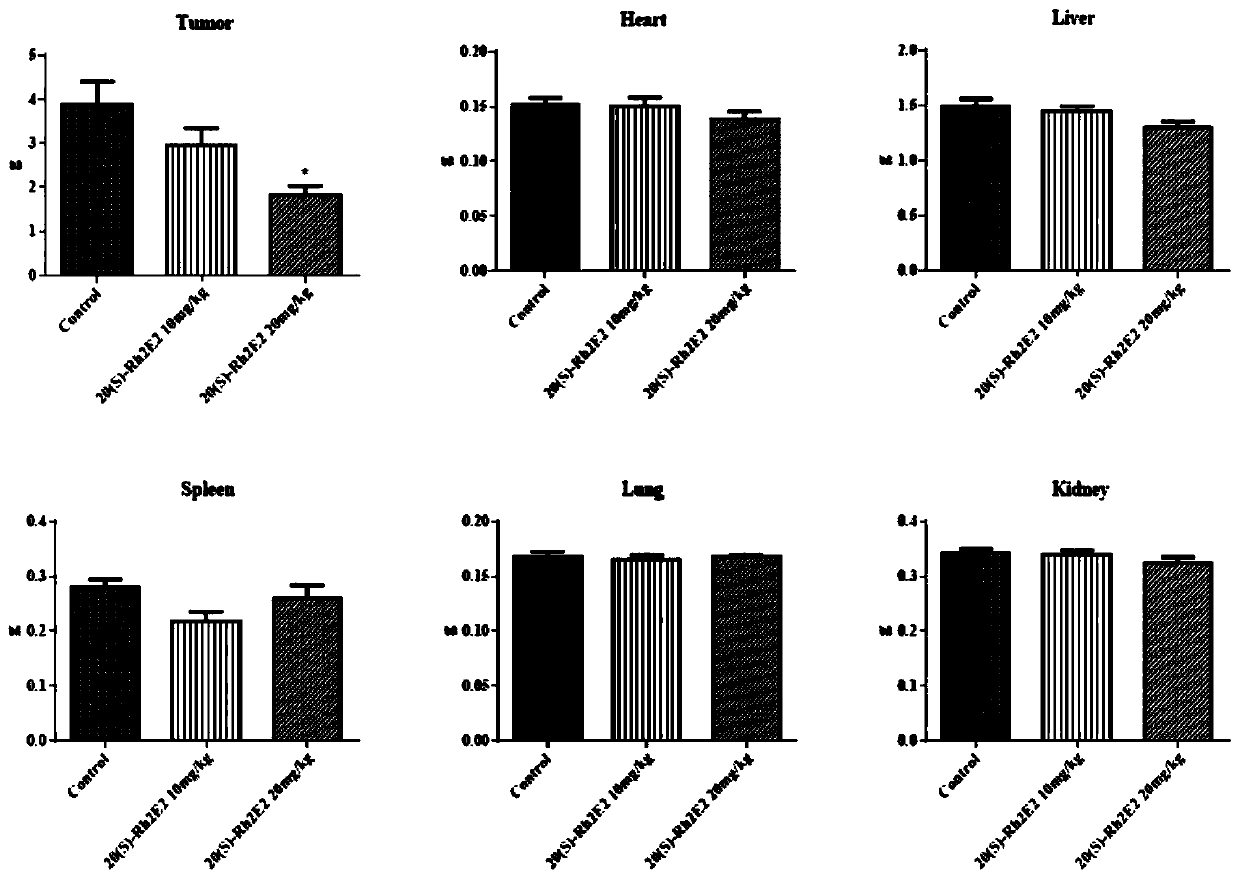
[0053] Effect of ginsenoside derivative 20(S)-Rh2E2 on inhibiting the growth of LLC-1 xenograft tumor
[0054] Male C57BL / 6 mice aged 6-8 weeks obtained from the Chinese University of Hong Kong (all animal care and experiments were approved by the Animal Ethics Committee of the Health Supervision Department of the Macau Special Administrative Guideline), 51 mice were subcutaneously injected with mouse lung cancer tumor LLC-1, and randomly divided into 5 groups. The ginsenoside derivative 20(S)-Rh2E2 prepared in Example 1 was dissolved in a mixed solution (wherein polyethylene glycol 400 (PEG400):ethanol:ddH2O=6:1:3), intraperitoneally injected, the dose was 10mg / kg and 20mg / kg, continuous injection for 21 days; and set up a group of control group (vehicle group) and a group of 20(S)-Rh2 group (dose is 10mg / kg), measure body weight and tumor volume (length×width) every day 2×1 / 2).
[0055] Such as figure 1 As shown, compared with the vhicle group, the 20(S)-Rh2E2 group inje...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap