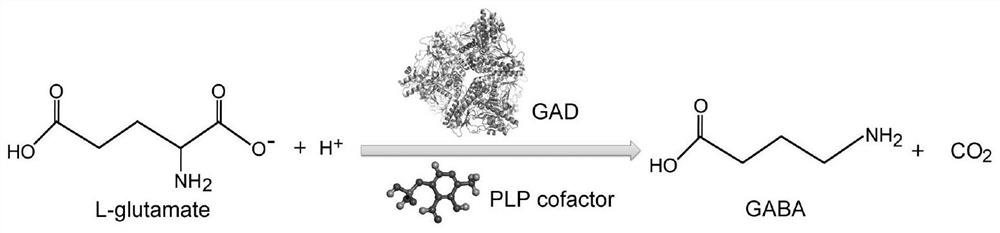
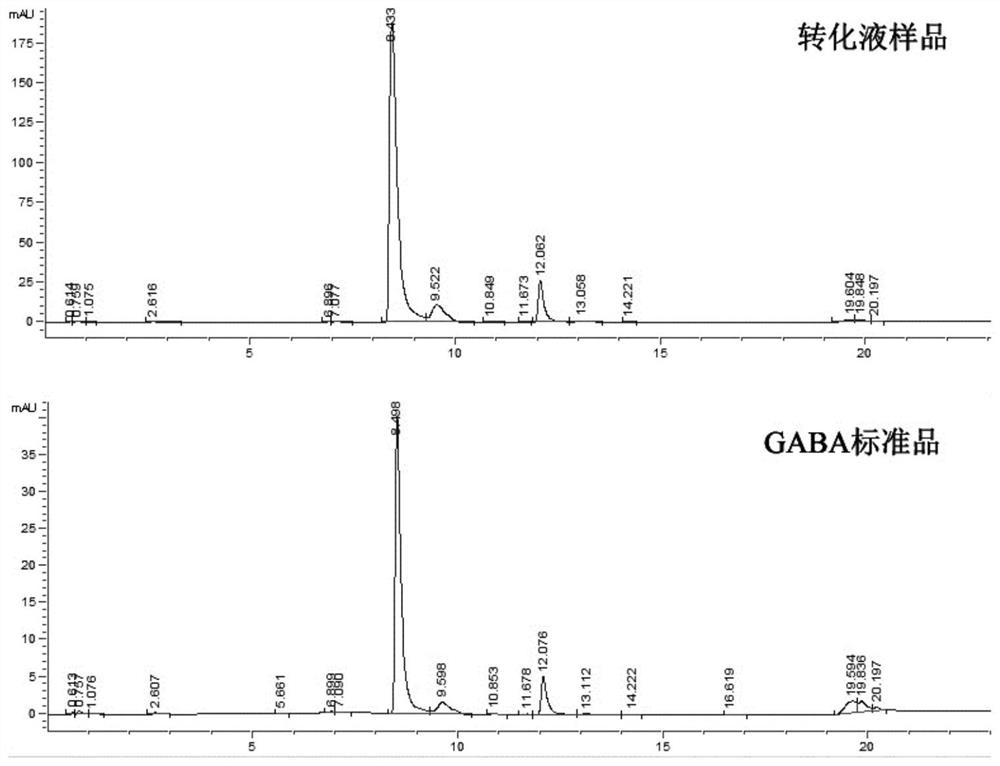
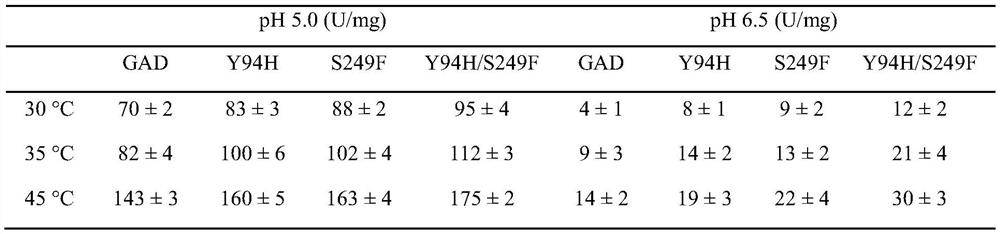
Glutamate decarboxylase mutant and application thereof in preparation of gamma-aminobutyric acid
A technology of glutamic acid decarboxylase and glutamic acid oxidase, which is applied in the field of bioengineering, can solve the problems of unknown enzyme activity and achieve the effects of avoiding food safety hazards, high comprehensive utilization of raw materials, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The construction of embodiment 1 glutamic acid decarboxylase mutant
[0016] By analyzing the alignment structure of the wild-type glutamic acid decarboxylase sequence and homologous sequences derived from other strains, combined with homology modeling and active site prediction, the 94th tyrosine Y of the amino acid sequence of SEQ ID NO:1 was determined And the 249th serine S is the mutation target. The site-directed mutagenesis strategy was adopted, and the point mutation primers were designed according to the amino acid site to be mutated, and the mutant sequence of glutamate decarboxylase was obtained by PCR.
[0017] Entrusted Suzhou Jinweizhi Biotechnology Co., Ltd. to synthesize the glutamic acid decarboxylase gene of Bacillus megaterium, and subcloned it into the pET21 expression plasmid based on the NdeI / HindIII double restriction site, and the recombinant plasmid was named pET21-GAD. Use the Tiangen Rapid Site-directed Mutagenesis Kit to carry out mutations ...
Embodiment 2
[0021] The enzyme activity assay of embodiment 2 glutamic acid decarboxylase mutants
[0022] The recombinant Escherichia coli obtained above containing the pET21-GAD, pET21-GAD-Y94H and pET21-GAD-S249F plasmids respectively were subjected to protein induction and expression. The specific method was: pick single clones and inoculate them in 100mL LB liquid medium (5g / L yeast extract, 10g / L tryptone, 10g / L NaCl), for overnight culture. Subsequently, according to the starting OD 600 ≈ 0.1 Transfer the seed solution to 100mL LB liquid medium, and wait until the cell concentration OD 600 At about 0.6, add a final concentration of 0.4mM IPTG to induce the expression of the target protein, adjust the culture temperature to 28°C, and continue the induction culture for 12h. After the induction is completed, the bacterial liquid is subjected to high-pressure homogenization and crushing treatment, and the purified glutamic acid decarboxylase protein is obtained by using a laboratory ...
Embodiment 3
[0027] Example 3 Construction of recombinant strains containing glutamic acid decarboxylase mutants
[0028] The host bacterial strain mentioned in the present invention is a bacterial strain that can express the glutamic acid decarboxylase mutant, and the host bacterial strain can be selected from Corynebacterium, Escherichia or Bacillus, for example, glutamic acid rod Bacillus (Corynebacterium glutamicum), Escherichia coli (Escherichia coli) or Bacillus subtilis (Bacillus subtilis), etc. One of the preferred hosts provided in this example is Escherichia coli BL21 and Corynebacterium glutamicum ATCC 13032 respectively.
[0029] Competent Escherichia coli BL21 and Corynebacterium glutamicum ATCC13032 were prepared by conventional laboratory standard methods. The pET21-GAD, pET21-Y94H, pET21-S249F, pET21-Y94H / S249F plasmids were transformed into Escherichia coli BL21 competent, respectively, and E. coli recombinant bacteria BL21-GAD, BL21-Y94H, BL21-Y94H, BL21-S249F and BL21-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com