Avian influenza HA-Fd fusion protein, and preparation method and vaccine thereof
A fusion protein and avian influenza technology, applied in the field of biomedicine, can solve the problems of poor immune activity and achieve good immunogenicity, good protection, and high-efficiency and quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In some optional embodiments, the preparation method includes the following steps:
[0033] (a) Codon-optimizing the gene encoding the avian influenza HA-Fd fusion protein and synthesizing the optimized gene.
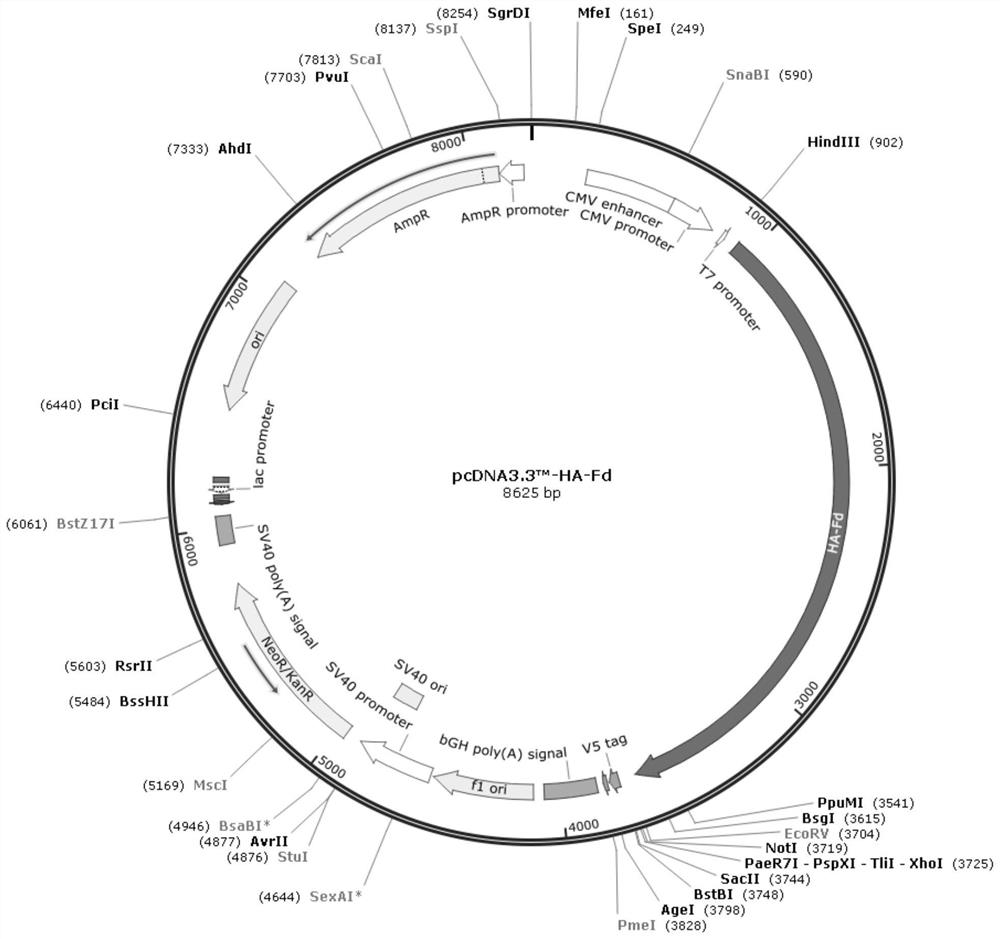
[0034] (b) Insert the artificially synthesized fusion protein gene into the eukaryotic expression vector pcDNA TM In 3.3-TOPO, positive recombinant vectors were screened out through enzyme digestion identification and sequencing, and the pcDNA3.3-HA-Fd plasmid was obtained.
[0035] (c) pcDNA3.3-HA-Fd plasmid, using FreeStyle TM MAX Reagent was transfected into CHO-S cells and named as CHO-HA-Fd. The avian influenza HA-Fd fusion protein was transiently expressed in CHO cells, and the cell supernatant containing the avian influenza HA-Fd fusion protein was obtained after purification, and stored at 4°C for future use.
[0036] pcDNA TM 3.3-TOPO is a bicistronic cloning vector, which can be applied to a variety of mammalian cell expression systems, using pcDNA ...
Embodiment 1
[0048] pcDNA TM 3.3-HA vector construction:
[0049] 1.1 Gene synthesis of avian influenza HA-Fd:
[0050] Obtain the avian influenza HA gene, GenBank accession number: ABW90137.1.
[0051] The HA gene was spliced with the Fd sequence, and the codon-optimized nucleotide sequence was optimized as shown in SEQID NO.4, and then a His tag with 6 histidines was added to the N-terminal, and a His tag was added at both ends of the gene. The ends were inserted into restriction sites HindIII and BamHI, and sent to a synthetic company for gene synthesis.
[0052] 1.2 pcDNA TM 3.3-HA-Fd plasmid construction:
[0053] 1.2.1 Ligation reaction: Ligation of the recovered PCR product and pcDNA3.3, the ligation system (10 μL / tube) is as follows:
[0054] pcDNA3.3 vector 1μL Recovery of digested products 2μL wxya 2 o
2μL Solution I 5μL
[0055] Ligation procedure: connect overnight on the ligation instrument at 16°C.
[0056] 1.2.2 Transformatio...
Embodiment 2
[0063] Preparation of HA-Fd trimer fusion protein by CHO expression system:
[0064] 1.1 CHO-S cell culture: medium: Hycell+8mM GlutaMAX; medium purchased from HyClone; CHO-S cell culture conditions: 37°C, 5% CO 2 , 125rpm; cultivated to a cell density of 1.5×10 6 / ml, transfection when cell viability ≥ 90%, cell density and viability are counted and observed by trypan blue staining, and transfection reagent is FreeStyle TM MAX Reagents, FreeStyle TM MAX reagent was purchased from Invitrogen, and cultured in a shaking shaker incubator after transfection, culture parameters: 37°C, 5% CO 2 , 125rpm.
[0065] 1.2 Plasmid extraction: using PureLink TM Hipure Plasmid Maxiprep Kit (endotoxin-free) kit for large plasmid extraction, please refer to the kit manual for the extraction method.
[0066] 1.3 Transfection: Using FreeStyle TM MAX Reagent was used as a transfection reagent to convert pcDNA TM 3. The 3-HA-Fd plasmid was transfected into CHO-S cells, and after 14 days ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com