In-situ hydrogel composition taking nano-micelles as cross-linking agent and application of in-situ hydrogel composition
A technology of hydrogel and composition, applied in the field of in-situ hydrogel composition, can solve the problems of low drug loading, difficult administration, limited number of active groups, etc., so as to reduce the toxic and side effects of drugs and reduce drug administration frequency, the effect of improving the photothermal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Preparation of base-catalyzed F127-HA micellar hydrogel composition and characterization of its physical and chemical properties
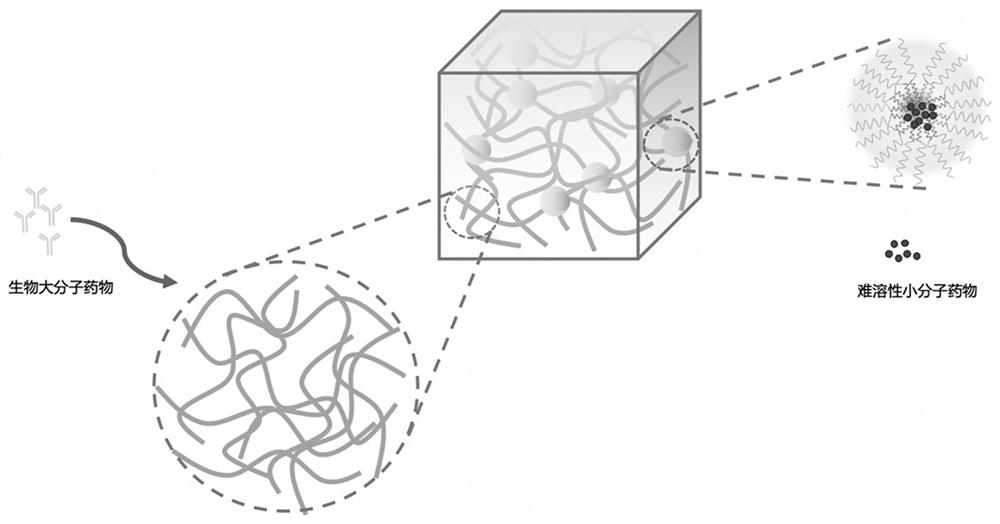
[0059] Schematic diagram of the structure of the micellar hydrogel figure 1 shown.
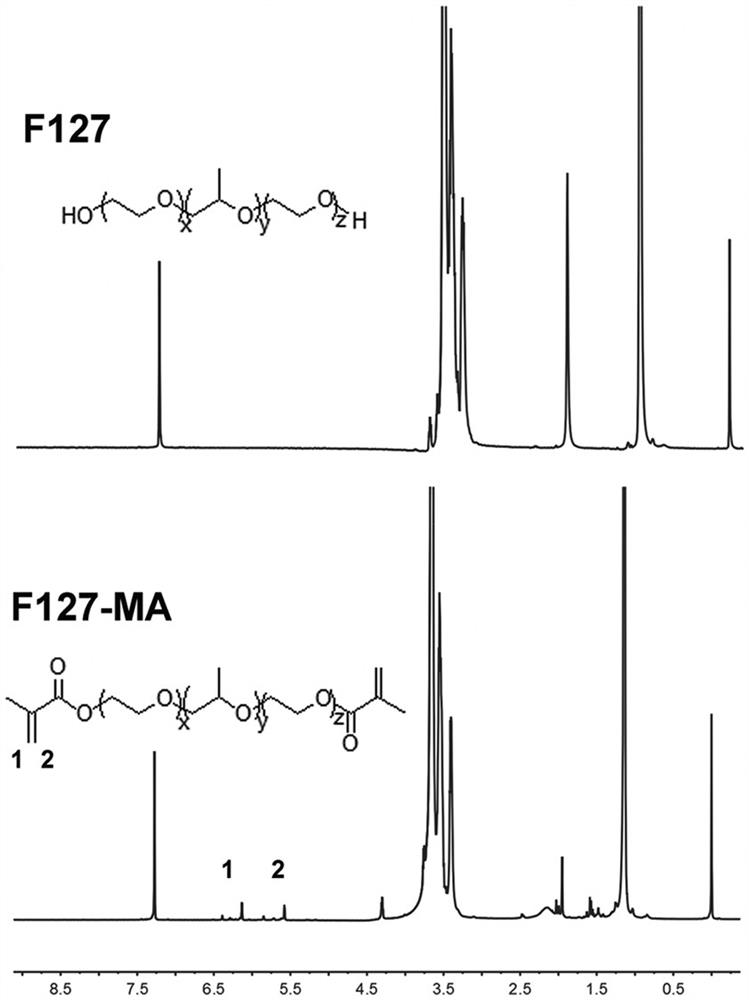
[0060] First, synthesize F127 methacrylate (bis-F127-MA), the structural formula and NMR spectrum are as follows figure 2 shown. Weigh 5 g (0.4 mmol) of Pluronic F127 (F127), dissolve it in 100 mL of anhydrous dichloromethane, add 2 mL of triethylamine and 4 mL of methacrylic anhydride ( 0.026 mol), and reacted at room temperature for 24 hours. After the reaction, the reaction solution was concentrated under reduced pressure to 20 mL, precipitated and crystallized with glacial ether with 5 times the volume of the reaction solution, filtered by suction, and dried in vacuum to obtain double bond-modified F127 (bis- F127-MA, 4.2 g, 80% yield). 1 HNMR (400 MHz, CDCl3, δ): 6.13 (s, 2H), 5.58 (s, 2H), 3.57 (bs, PEG), 1.13 (bs, 6H) ( figure 2 ). ...
Embodiment 2
[0063] Example 2: Preparation of alkali-catalyzed F127-HA micellar hydrogel composition loaded with tripterine
[0064] Weigh 10 mg of tripterine (CLT) and dissolve in 10 mL of acetonitrile to prepare a 1 mg / mL stock solution. Weigh 50 mg of bis-F127-MA, dissolve it in 5 mL of acetonitrile, add 3 mL of CLT stock solution, mix well, then rotate and evaporate to form a drug-containing polymer film, and then disperse in 2 mL of HEPES (pH8.0, 0.1M) buffer CLT-loaded F127-MA micelles were obtained. The micelles prepared by the above method were spherical and uniform in size, with an average particle diameter of 35.6 ± 0.6 nm, a PDI of 0.168 ± 0.02, a zeta potential of -0.13 ± 0.011mV, and an encapsulation efficiency of (95.4 ± 2.0)%. The dose was (1.62 ± 0.1)%. F127-MA micelles TEM image and particle size image as shown Figure 5 shown.
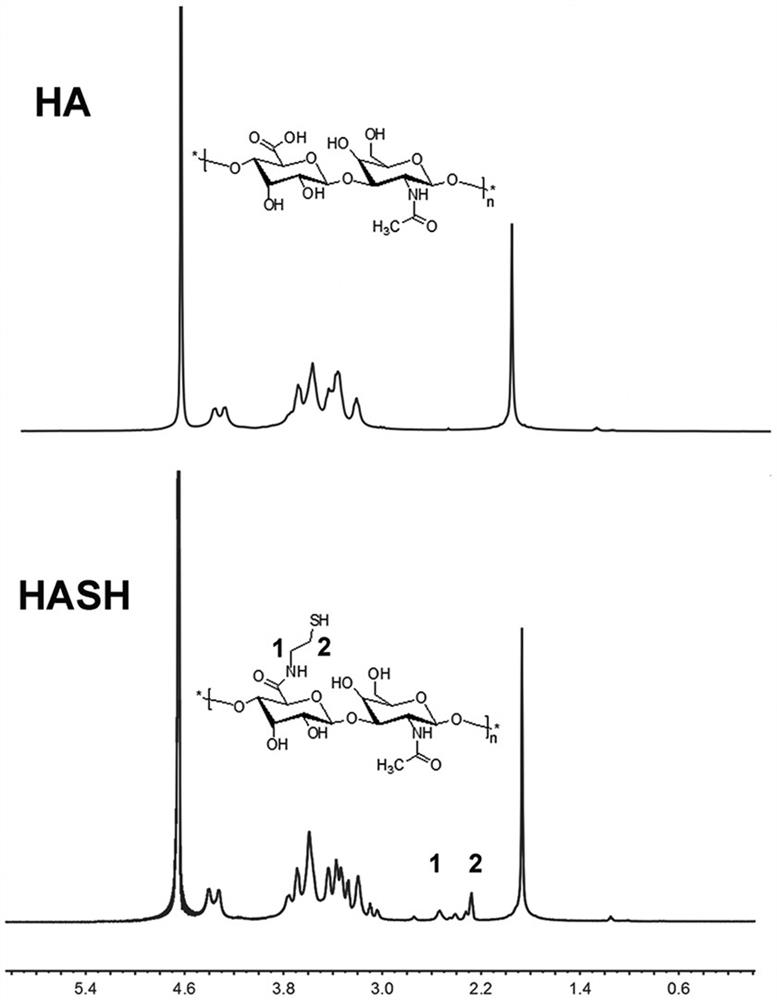
[0065] Prepare drug-loaded micelles (5% - 10%) with 1M, pH8.0 HEPES as solvent, and prepare 1% - 10% solution by dissolving thiol-modified hy...
Embodiment 3
[0066] Example 3: Preparation of the base-catalyzed F127-HA micellar hydrogel composition loaded with prednisolone
[0067] Weigh 10 mg of prednisolone (PRE) and dissolve in 10 mL of acetonitrile to prepare a 1 mg / mL stock solution. Weigh 50 mg of bis-F127-MA, dissolve it in 5 mL of acetonitrile, add 3 mL of PRE stock solution, mix well, then rotatively evaporate to form a drug-containing polymer film, and then disperse in 2 mL of HEPES (pH8.0, 0.1M) buffer Preloaded F127-MA micelles. The micelles are spherical in shape, uniform in particle size distribution, and the encapsulation efficiency is greater than 90%. In the same way, 10% HASH solution was prepared with pure water as solvent, and 2 mL of HASH solution was mixed with 2 mL of PRE-loaded micellar solution in equal volume. The glue is uniform and transparent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com