Hansenula polymorpha engineering bacteria for efficiently expressing CA10 virus-like particles and application thereof
A high-efficiency expression, Hansenula yeast technology, applied in the field of biomedicine, can solve the problems of difficult proportion of solid virus particles, virulence recovery, incomplete inactivation, etc., and achieve easy large-scale production, simple process operation, and high antigen recovery rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
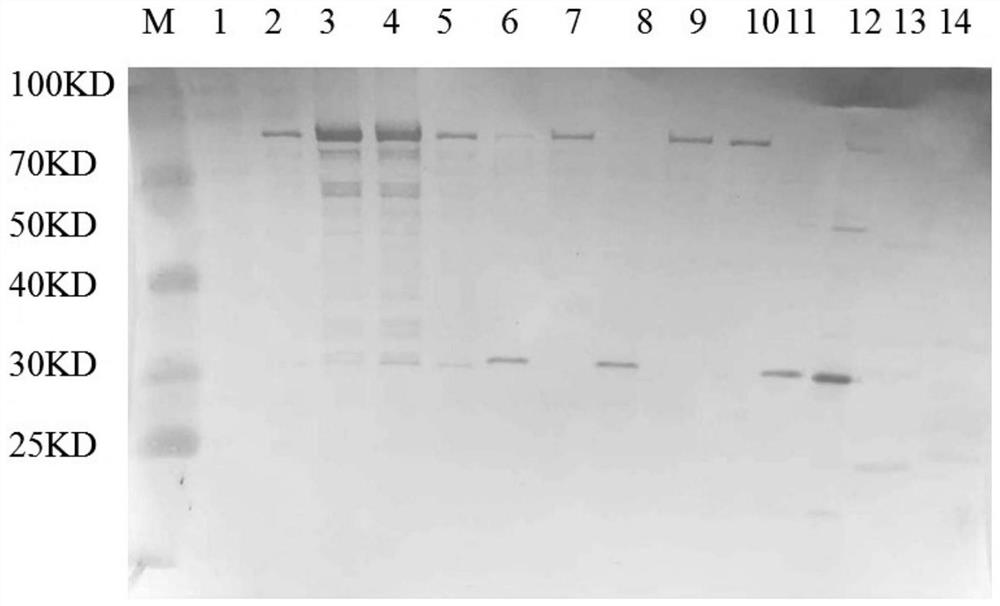
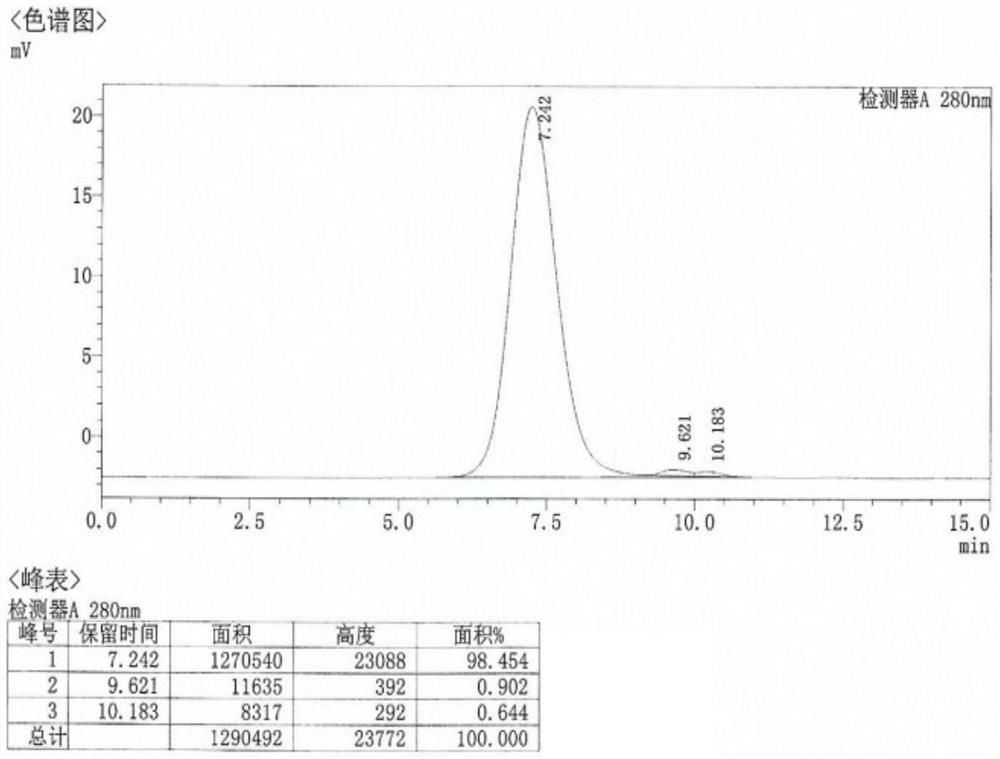
[0068] The invention provides a method for preparing and purifying CA10 virus-like particles, and an application in the field of vaccines. Through the high-density fermentation culture of recombinantly expressed Hansenula engineering bacteria and the expression of CA10 virus-like particle protein induced by methanol, the bacteria were collected by centrifugation and subjected to high-pressure homogenization and crushing. The supernatant was subjected to ultrafiltration, ion exchange chromatography, and hydroxyapatite. Obtained after purification by chromatography and molecular sieve chromatography. The CA10 virus-like particle provided by the present invention and the vaccine prepared therefrom have good immunogenicity, safety, immune characteristics and biological activity, simple process, and chromatography method is adopted for purification, which is more conducive to linear amplification than density gradient centrifugation. It can be prepared and purified on a large scale...
Embodiment 1
[0083] Example 1 The acquisition of CA10 recombinant Hansenula engineering bacteria
[0084] According to the nucleotide sequences of the P1 and 3CD proteins of the recently popular Coxsackievirus A10 strain, Vector software was used to optimize the design of the P1 and 3CD gene sequences according to the preferred codons of Hansenula to increase their expression.
[0085] The sequence of the yeast secretion signal peptide or the sequence of the transcription termination signal recognized by yeast was removed during the optimization process. In order to avoid the GC content of the translated mRNA being too high, the secondary structure of the mRNA affecting the translation efficiency, and considering the enzyme cutting site, the gene sequence at certain positions was adjusted appropriately to ensure that the amino acid remained unchanged. The nucleotide sequences of the optimized CA10 virus P1 and 3CD genes are shown in SEQ ID NO: 1 and 2, respectively.
[0086] In the presen...
Embodiment 2
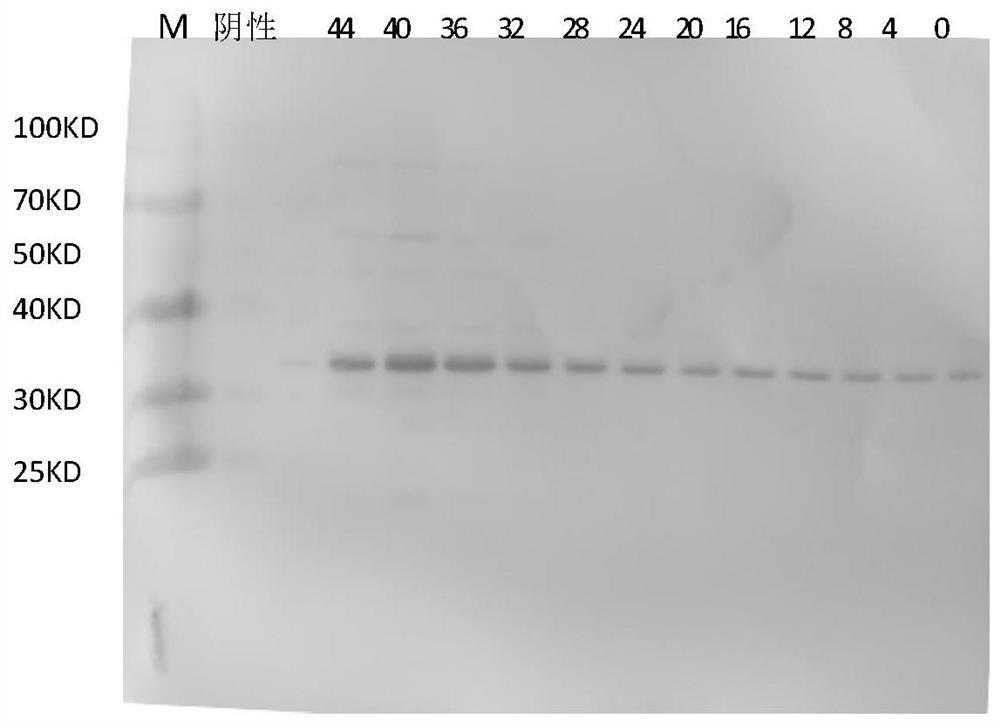
[0091] Embodiment 2 Recombinant CA10 yeast expression strain is fermented and cultivated in a 30L fermenter
[0092] Strain CA10-W was inoculated into 100ml primary seed medium (0.67% yeast nitrogen source medium, 0.5% ammonium sulfate, 2% glucose), and cultured at 33°C and 200rpm on a shaker for 18-22h. Take the primary seed culture solution and inoculate it into 1000ml of the secondary seed medium, and incubate at 33°C and 200rpm on a shaker for 21 to 23 hours. Inoculate the secondary seed culture liquid into a 30L fermenter, adjust the pH value of the fermentation liquid with ammonia water to maintain at 5.0+0.5, the fermentation temperature is 30±1°C, the rotation speed is controlled at 350-750rpm, and the air flow rate is 0.5-1.0m 3 / h, high-density fermentation requires pure oxygen supplementation, dissolved oxygen is controlled at 20-60%, the carbon source in the fermentation medium is exhausted in 16-18 hours, and a total of 2.0L of glycerin is added, and the total gro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| crush indicators | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com