Hydrogen peroxide responsive polymer nano-carrier with fluorescence ratio characteristic as well as preparation method and application thereof
A hydrogen peroxide and nanocarrier technology, applied in the field of hydrogen peroxide responsive synthetic polymers, can solve the problems of reduced treatment results, multidrug resistance, low solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
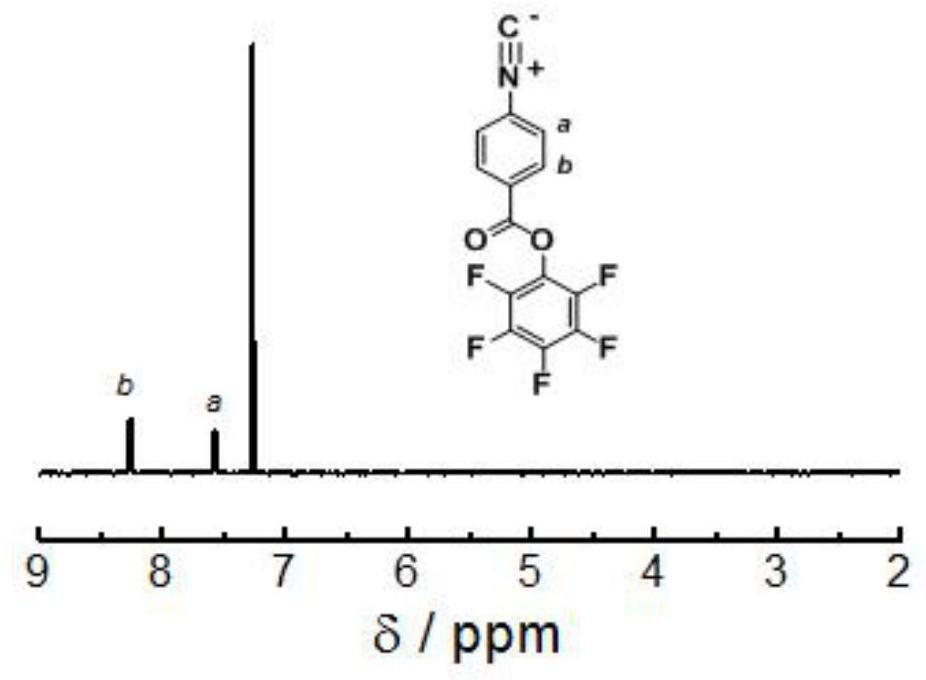
[0109] Implementation Example 1: Preparation of Pentafluorophenol Isonitrile Monomer
[0110] Add p-nitrobenzoic acid (1.1eq), pentafluorophenol (1eq), EDCI (1.2eq), and DMAP (0.5eq) into a two-necked flask, use dichloromethane as a solvent, and react at room temperature. After the reaction is followed by TLC, use Wash 3 times with water, 3 times with saturated sodium bicarbonate solution, 3 times with saturated sodium chloride solution, collect the organic phase, add anhydrous sodium sulfate to dry for 3 hours, remove anhydrous sodium sulfate by suction filtration, remove the solvent by rotary evaporation, pass through a silica gel column After purification (eluent: petroleum ether: ethyl acetate = 1:1), the product was collected, the solvent was removed, and vacuum-dried to constant weight. After passing through a silica gel column (DCM:PE=1:1), the product was collected, spin-dried and then vacuum-dried to constant weight to obtain product I, the structural formula of which...
Embodiment 2
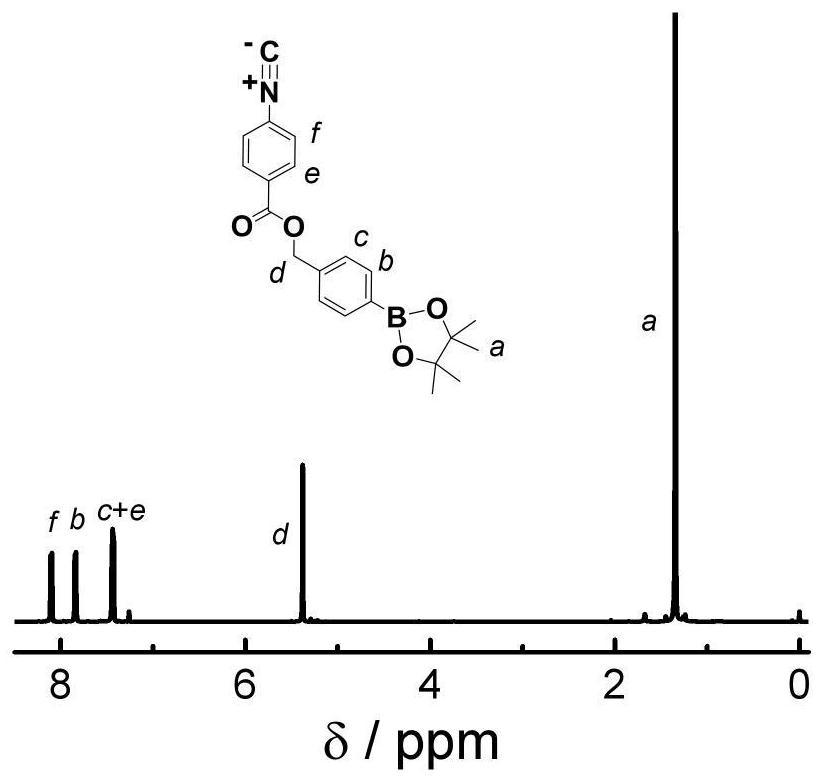
[0120] Implementation Example 2: Preparation of Benzene Isonitrile Monomer with Hydrogen Peroxide Response
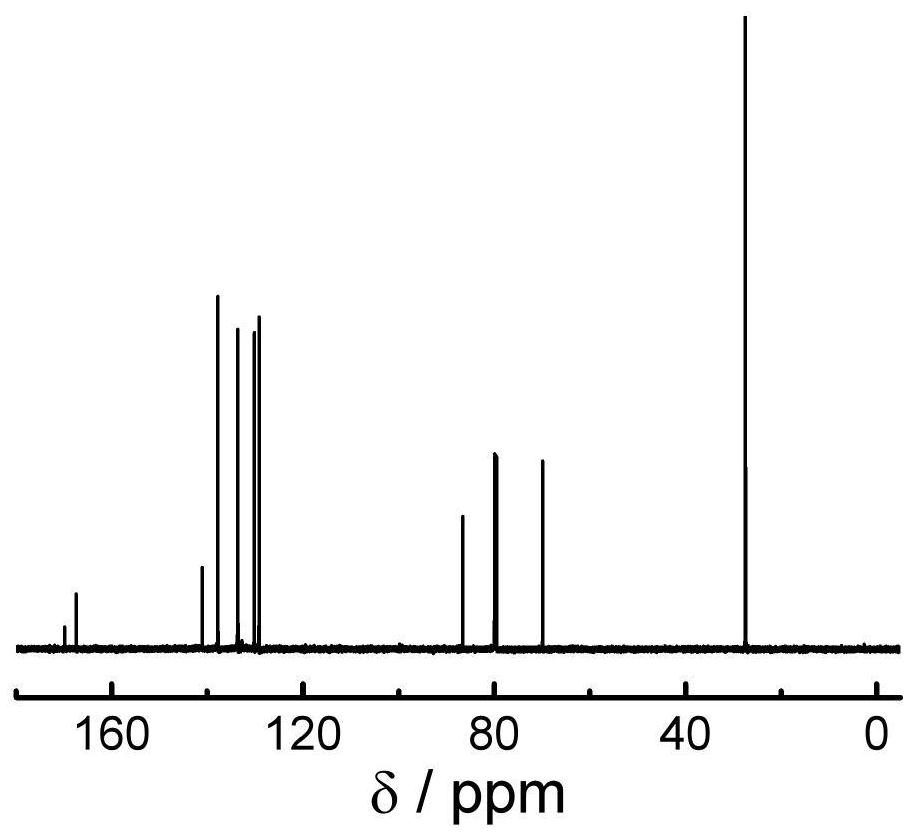
[0121] Add pentafluorophenol isonitrile (1eq), 4-hydroxymethylphenylboronic acid pinacol ester (1.1eq), DMAP (1.2eq), and anhydrous THF into a two-necked bottle, vacuum-fill with nitrogen, repeat 3 times , under a nitrogen atmosphere, stirred at 30°C for reaction, followed by TLC and spin-dried after the completion of the reaction, passed through a silica gel column (DCM:PE=9:1), collected the product, spin-dried, vacuum-dried to constant weight, and stored in a freezer. The synthetic route is as follows:
[0122]
Embodiment 3
[0123] Implementation Example 3: Preparation of tetraphenylethylene benzene isocyanate monomer
[0124] Add zinc powder (4.2eq), 4-hydroxybenzophenone (1eq), and diphenylketone (1eq) into the two-necked bottle, and make the two-necked bottle pass through the N 2 Degas for 30 min, then add anhydrous THF as solvent, cool the mixture to -20 °C, slowly inject TiCl 4 (2eq). The mixture was stirred at -20°C for 0.5 hours, then refluxed at 55°C overnight. After passing K 2 CO 3 Aqueous solution (10%) quenched the reaction, and extracted with ether, combined the organic phases and washed three times with brine, and the mixture was washed with anhydrous Na 2 SO 4 Dry, pass through a silica gel column (DCM:petroleum ether=4:1), collect the product, remove the solvent, spin dry and vacuum-dry to constant weight to obtain product I, the structural formula is:
[0125]
[0126] Add 4-nitrobenzoic acid (2.2eq), EDCI (2.2eq), DMAP (1eq) into a two-necked flask, anhydrous CH 2 Cl 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com