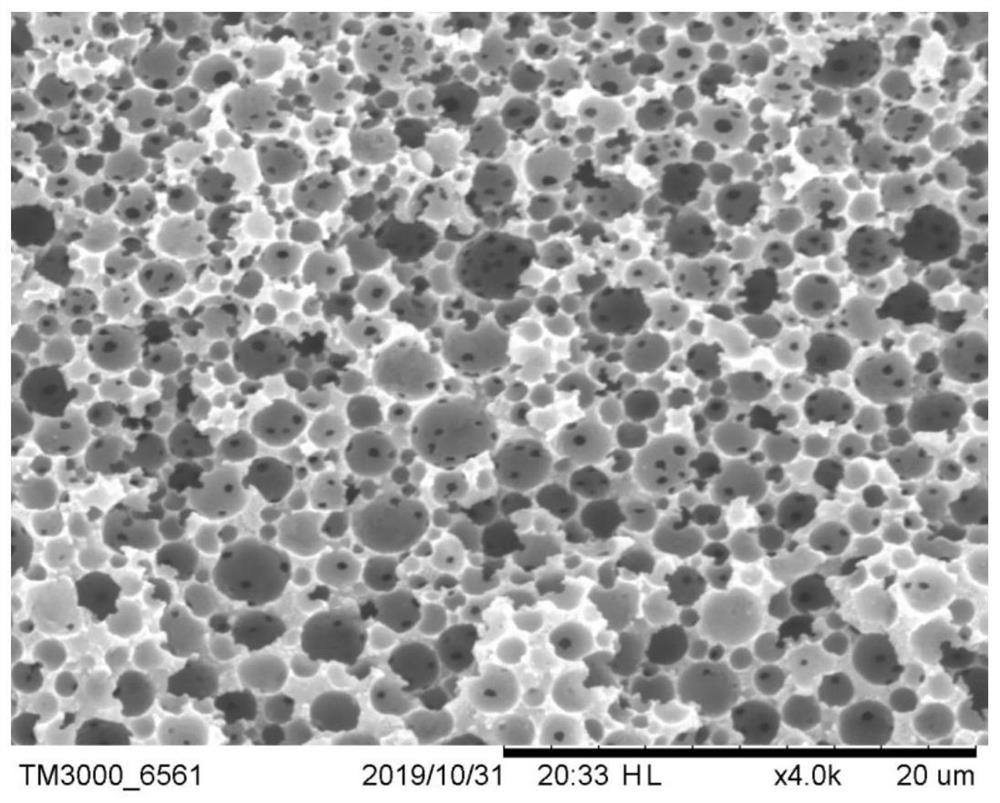
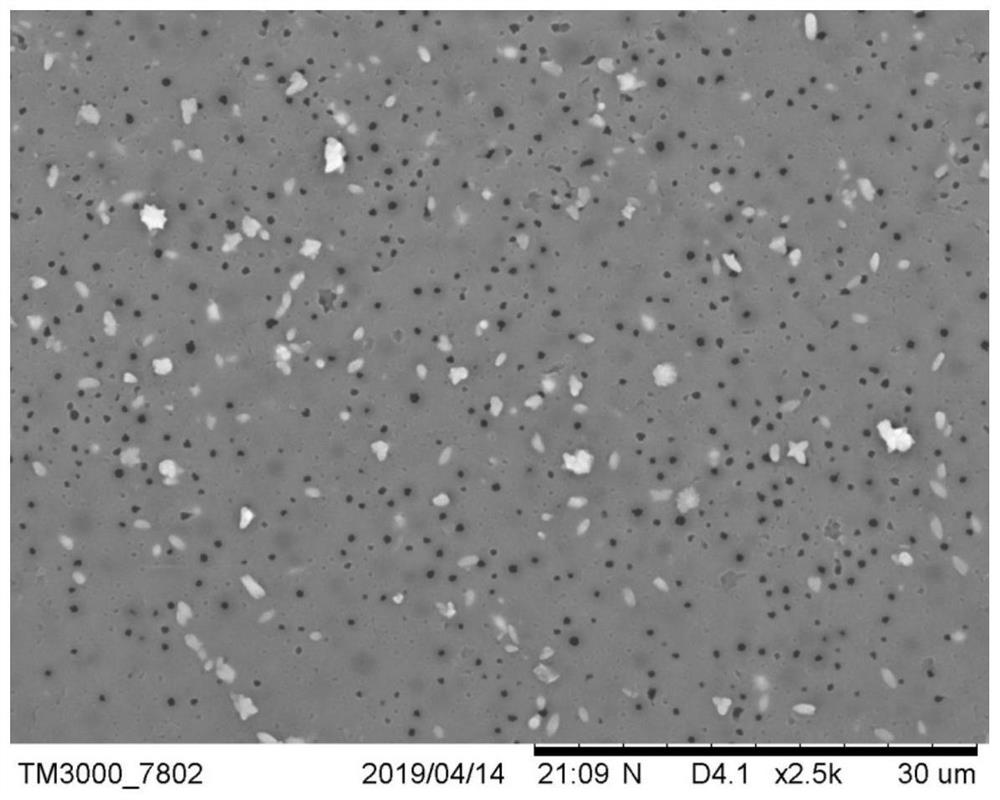
Polyacrylate composite material for CO2 adsorption and preparation method of polyacrylate composite material
A polyacrylate, composite material technology, applied in separation methods, chemical instruments and methods, other chemical processes, etc., can solve problems such as being unsuitable for fluidized beds, easy to block resin pores, and insufficient stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Put 0.4g methyl methacrylate, 0.4g tert-butyl methacrylate, 0.7g glycidyl methacrylate and 1g trimethylolpropane triacrylate into a flask, add 3ml of toluene and 0.3 g of polyoxypropylene-polyoxyethylene copolymer, add 10ml of deionized water dropwise into the flask at a speed of 1000r / min, continue stirring for 10min after the dropwise addition, to obtain a stable emulsion, take out the emulsion and add 0.1g of benzyl peroxide Stir at a high speed of 5000r / min for 2min;
[0037] (2) Take 150ml of deionized water and add 0.3g of polyvinyl alcohol to make a dispersed phase, add the emulsion to the dispersed phase and disperse into balls at a stirring speed of 250r / min, add 6 drops of N, N, N after stabilization ',N'-Tetramethylethylenediamine is used as a reducing agent. After solidification, the balls are taken out and dried in an oven at 70°C for 2 hours to obtain spherical particles with a particle size of 0.3-1.0mm;
[0038] (3) Take 2.5g of spherical particles,...
Embodiment 2
[0040] (1) Put 0.2g methyl methacrylate, 0.4g tert-butyl methacrylate, 0.9g glycidyl methacrylate and 1g trimethylolpropane triacrylate into a flask, add 3ml of toluene and 0.3 g of polyoxypropylene-polyoxyethylene copolymer, add 10ml of deionized water dropwise into the flask at a speed of 1000r / min, continue stirring for 10min after the dropwise addition, to obtain a stable emulsion, take out the emulsion and add 0.1g of benzyl peroxide Stir at a high speed of 5000r / min for 2min;
[0041] (2) Take 150ml of deionized water and add 0.3g of polyvinyl alcohol to make a dispersed phase, add the emulsion to the dispersed phase and disperse into balls at a stirring speed of 250r / min, add 6 drops of N, N, N after stabilization ',N'-Tetramethylethylenediamine is used as a reducing agent. After solidification, the balls are taken out and dried in an oven at 70°C for 2 hours to obtain spherical particles with a particle size of 0.3-1.0mm;
[0042] (3) Take 2.5g of spherical particles,...
Embodiment 3
[0044] (1) Put 0.4g methyl methacrylate, 0.2g tert-butyl methacrylate, 0.9g glycidyl methacrylate and 1g trimethylolpropane triacrylate into a flask, add 3ml of toluene and 0.3 g of polyoxypropylene-polyoxyethylene copolymer, add 10ml of deionized water dropwise into the flask at a speed of 1000r / min, continue stirring for 10min after the dropwise addition, to obtain a stable emulsion, take out the emulsion and add 0.1g of benzyl peroxide Stir at a high speed of 5000r / min for 2min;
[0045] (2) Take 150ml of deionized water and add 0.3g of polyvinyl alcohol to make a dispersed phase, add the emulsion to the dispersed phase and disperse into balls at a stirring speed of 250r / min, add 6 drops of N, N, N after stabilization ',N'-Tetramethylethylenediamine is used as a reducing agent. After solidification, the balls are taken out and dried in an oven at 70°C for 2 hours to obtain spherical particles with a particle size of 0.3-1.0mm;
[0046] (3) Take 2.5g of spherical particles,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com