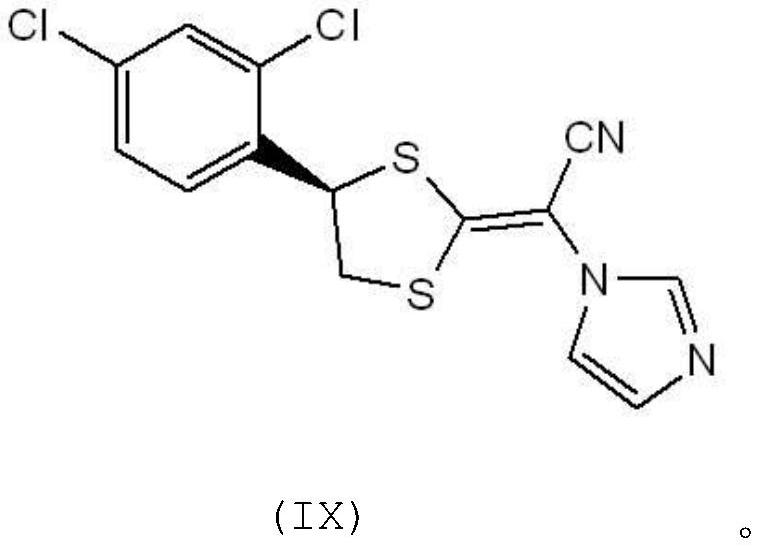
Preparation method of azole antifungal drug
A technology of imidazole and azole oil, which is applied in the field of drug preparation, can solve problems such as complex operation, easy decomposition, and easy deliquescent, and achieve the effects of simplifying the process, increasing the yield, and not easily absorbing water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
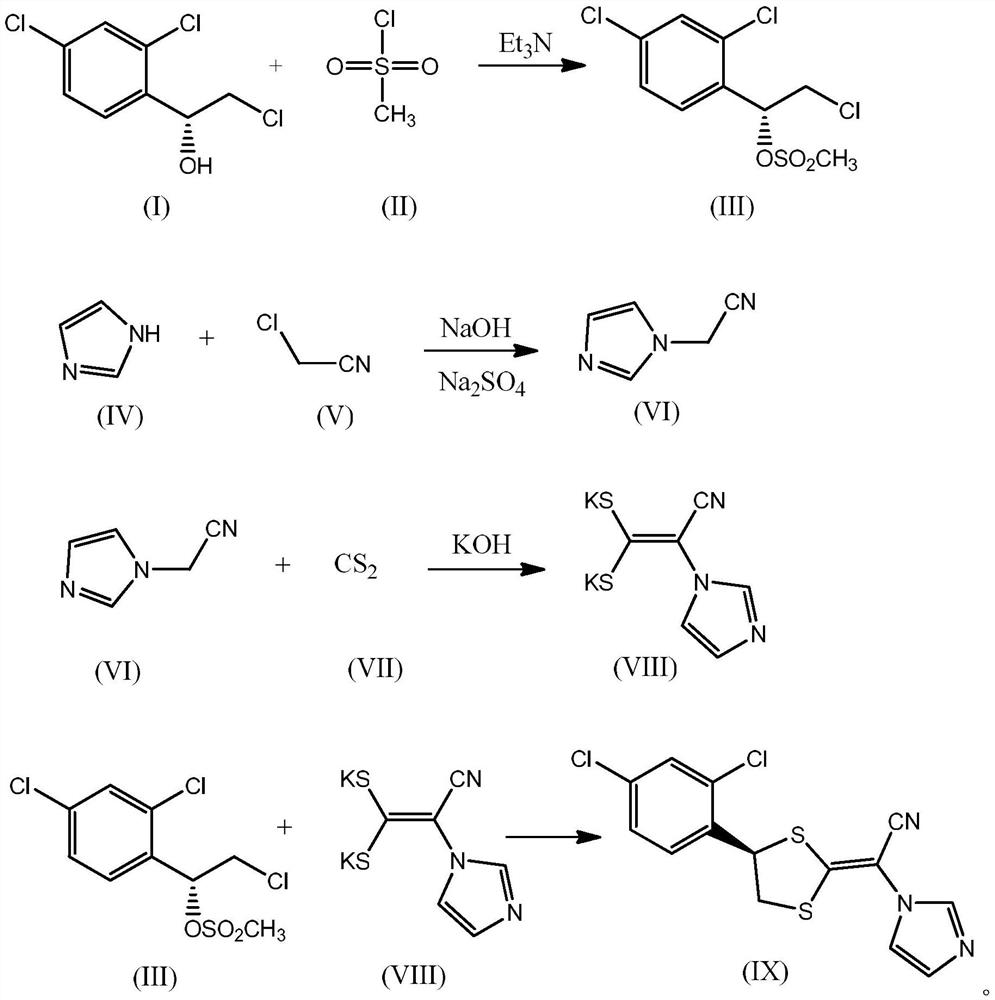
[0075] Embodiment 1, synthesis (S)-2-chloro-1-(2,4-dichlorophenyl) ethyl methanesulfonate
[0076]
[0077] Add 6 kg of dichloromethane into a 20-liter four-neck flask, add 2 kg of (S)-2-chloro-1-(2,4-dichlorophenyl)ethanol under stirring, and the inner temperature is 2°C. After adding 0.96 kg of triethylamine, keep the internal temperature at 0-5°C, add 1.06 kg of methanesulfonyl chloride dropwise, control the dropping time to 0.7 hours, and continue stirring for 20 minutes after the dropping is completed.
[0078] Add 2 kg of water to the reaction flask to terminate the reaction, separate the water phase, extract the water phase once with dichloromethane, combine the organic phase, wash the organic phase with 2 mol / L hydrochloric acid solution, wash with saturated sodium bicarbonate solution, and wash with water , adding anhydrous sodium sulfate and drying for 30 minutes.
[0079] The organic phase was distilled to collect 5.6 kg of dichloromethane fractions, and 3 kg of...
Embodiment 2
[0080] Embodiment 2, synthetic 1-(cyanomethyl) imidazoles
[0081]
[0082] Add 1900 grams of acetonitrile, 230 grams of anhydrous sodium sulfate solid, 508 grams of imidazole and 320 grams of sodium hydroxide powder into a 5-liter reaction flask; keep the inner temperature at -5 to 5°C and stir for 10 minutes.
[0083] Control the temperature at -5 to 5°C, add 608 grams of chloroacetonitrile dropwise to the above 5 liter reaction flask, the dropping time is controlled at 1.2 hours, after the dropping is completed, continue to stir and react for 10 minutes. After the stirring reaction finishes, remove the insoluble matter by filtration, the mother liquor is distilled and concentrated to obtain 1070 grams of oil, add 1500 grams of dichloromethane and 850 grams of toluene to the oil, stir for 10 minutes, and separate the organic phase after standing for 10 minutes , Distilled and concentrated to obtain 670 grams of 1-(cyanomethyl) imidazole oil, which was crystallized with 14...
Embodiment 3
[0084] Embodiment 3, synthetic intermediate (VIII)
[0085]
[0086] Add 450 grams of 1-(cyanomethyl) imidazole to a 10-liter reaction flask, add 5 kilograms of DMSO, and stir until a homogeneous solution; add 325 grams of carbon disulfide to the system; keep the internal temperature at 10-35°C, and distribute Add 480 grams of potassium hydroxide in batches, and keep stirring for 0.5 hours after adding.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com