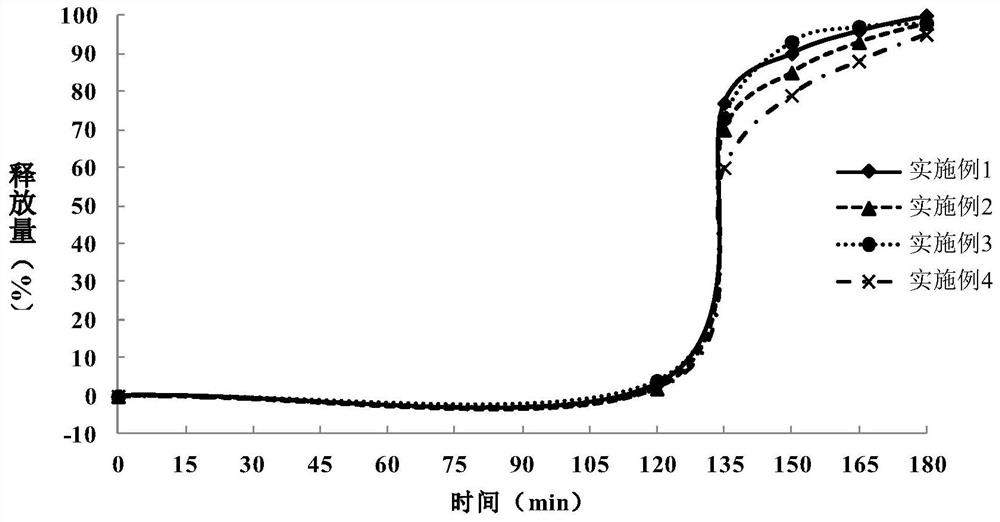
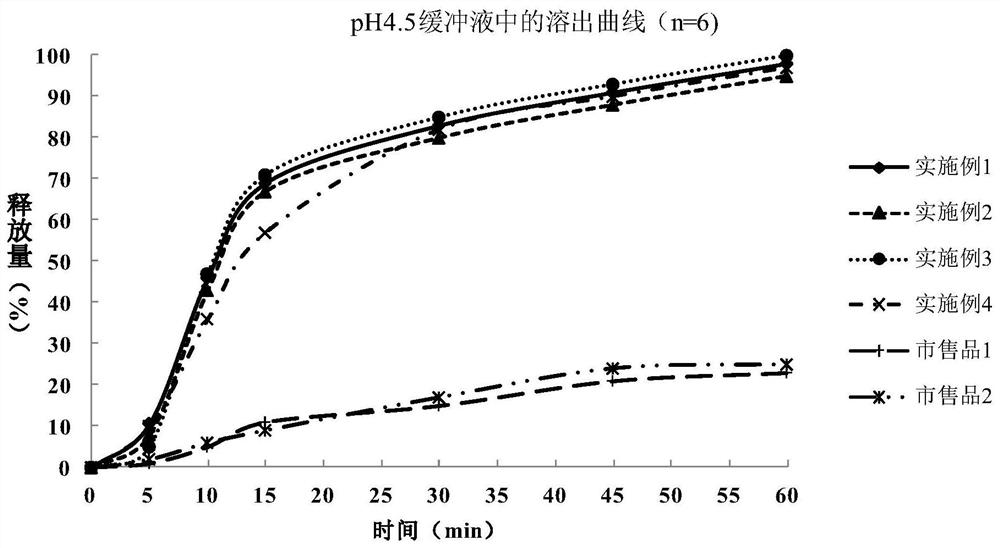
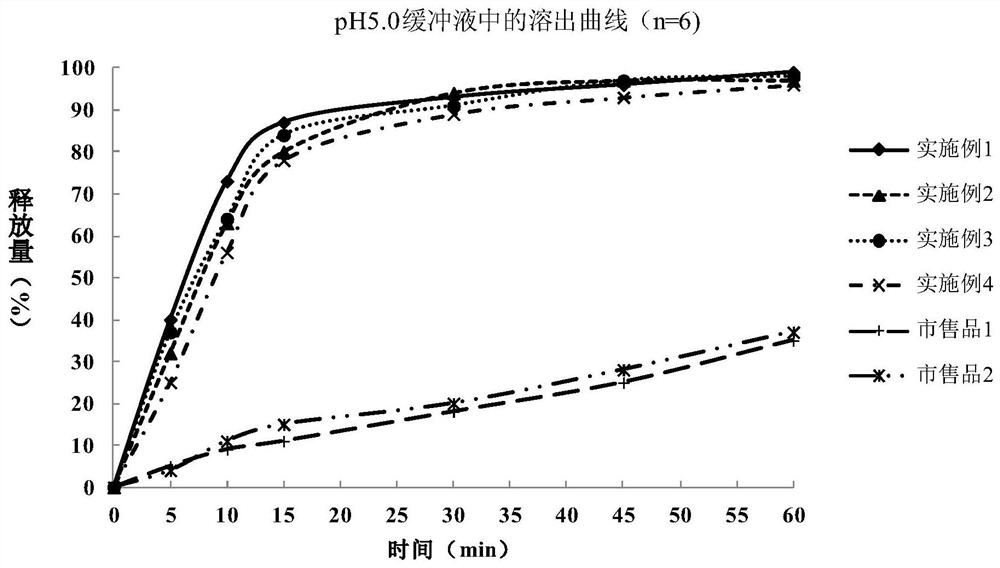
Metformin hydrochloride enteric-coated tablet and preparation method thereof
A metformin hydrochloride enteric and tablet-dissolving technology, which is applied in the field of medicine, can solve the problems of short retention and absorption time of metformin, unfavorable metformin absorption, and slow release rate, and achieve the effect of being suitable for industrial production, simple preparation method, and rapid release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Metformin Hydrochloride Enteric-Coated Tablets
[0067] Tablet prescription:
[0068] composition Dosage (g) Metformin Hydrochloride 500.0 Povidone 27.5 Crospovidone 16.5 Magnesium stearate 5.0 50% ethanol solution 20-30
[0069] Enteric coating solution prescription:
[0070]
[0071]
[0072] Preparation:
[0073] 1) Tablet core preparation: crush metformin hydrochloride through a 100-mesh sieve and set aside. The prescription auxiliary materials were weighed according to the prescription amount, metguanidine hydrochloride and povidone K29 / 32 were placed in a wet granulator and mixed evenly, and an appropriate amount of 50% ethanol aqueous solution was added to granulate. Place in an oven and dry until the water content is less than 3%, then add magnesium stearate for total blending, and compress into tablets to make tablet cores.
[0074] 2) Enteric coating: dissolve hypromellose phthalate in 70% ethanol aqueous...
Embodiment 2
[0076] Metformin Hydrochloride Enteric-Coated Tablets
[0077] Tablet prescription:
[0078] composition Dosage g Metformin Hydrochloride 500.0 Hypromellose 13.8 Crospovidone 11 Magnesium stearate 5.2 purified water 15-30
[0079] Enteric coating solution prescription:
[0080] composition Dosage g Methacrylic acid-ethyl acrylate copolymer 21.4 Propylene Glycol 1.27 talcum powder 2.15 60% ethanol in water 250.6
[0081] Referring to Example 1 preparation method.
Embodiment 3
[0083] Metformin Hydrochloride Enteric-Coated Tablets
[0084] Tablet prescription:
[0085] composition Dosage g Metformin Hydrochloride 500.0 Povidone 20.5 Carboxymethyl Starch Sodium 11 Sodium stearyl fumarate 5.2 65% ethanol solution 25-40
[0086] Enteric coating solution prescription:
[0087] composition Dosage g Methacrylic acid copolymer 30.3 triethyl citrate 2.39 talcum powder 4.09 80% ethanol in water 286.2
[0088] Referring to Example 1 preparation method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com