Method of preparing and purifying lithium sulfide
A lithium sulfide and lithium sulfate technology, applied in chemical instruments and methods, sulfur compounds, alkali metal sulfides/polysulfides, etc., can solve the problems of poor controllability of composite material morphology, difficulty in preparing lithium sulfide, and unstable product quality and other problems, to achieve the effect of easy industrial implementation, high yield and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The method for preparing and purifying lithium sulfide provided by a typical embodiment of the present invention includes
[0023] Step 1, dissolving lithium sulfate and soluble carbon source in deionized water in proportion, and then spray-drying and granulating to obtain powder.
[0024] The ratio of lithium sulfate and soluble carbon source is Li 2 SO 4 +2C=Li 2 S+2CO 2 Weighing, excess carbon source 3-5%.
[0025] Preferably, in step 1, the soluble carbon source is one or a combination of sucrose, glucose and citric acid.
[0026] Step 2: Calcining the granulated powder under the protection of an inert atmosphere.
[0027] Preferably, in step 2, the inert atmosphere is nitrogen, argon or hydrogen-argon mixed gas. The calcination temperature is 750~900℃, and the calcination time is 5~15h.
[0028] Step 3: In a dehumidifying and drying environment, the calcined powder is washed with absolute ethanol to filter out insoluble lithium sulfate and carbon powder, and...
Embodiment 1
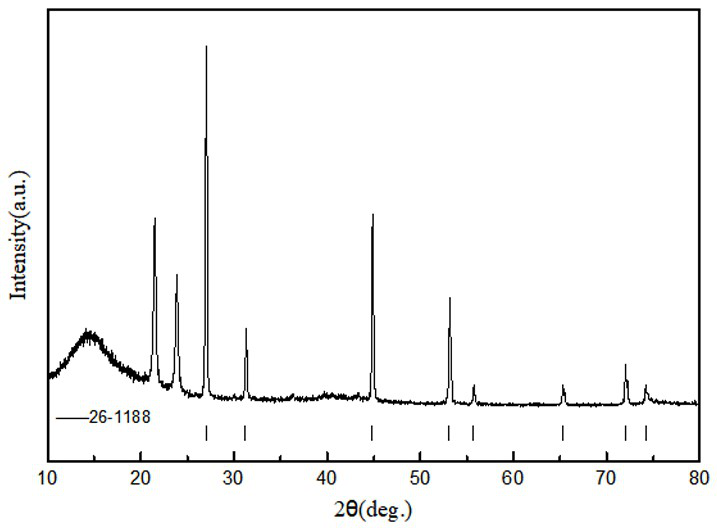
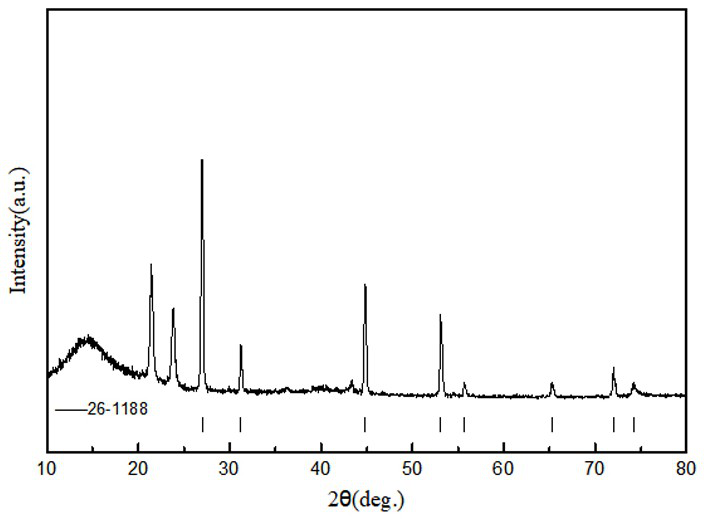
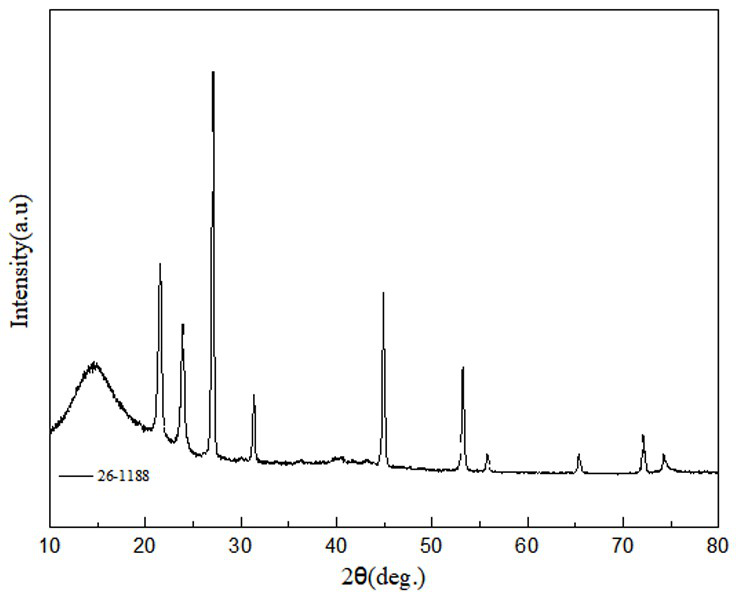
[0032] According to Li 2 SO 4 +2C=Li 2 S+2CO 2 For the reaction, lithium sulfate and sucrose were dissolved in deionized water in proportion (excess sucrose 5%), and then spray-dried and granulated; under the protection of nitrogen, the granulated powder was calcined in a tube furnace at 750°C for 15 hours; In a water anaerobic glove box (both water and oxygen content is lower than 1ppm), the calcined powder is washed with absolute ethanol to filter out insoluble lithium sulfate and carbon powder, and then the filtrate is evaporated, crystallized, purified and dried to obtain lithium sulfide , with a purity of 99.4%.
Embodiment 2
[0034] According to Li 2 SO 4 +2C=Li 2 S+2CO 2 Reaction, dissolve lithium sulfate and citric acid in proportion (5% citric acid excess) in deionized water, then spray dry and granulate; under the protection of hydrogen and argon mixed gas, the granulated powder is 900°C in a tube furnace Calcination for 5 hours; in the dehumidification and drying room (water content less than 100ppm), the calcined powder is washed with absolute ethanol to filter out insoluble lithium sulfate and carbon powder, and then the filtrate is evaporated, crystallized, purified and dried to obtain lithium sulfide , with a purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com