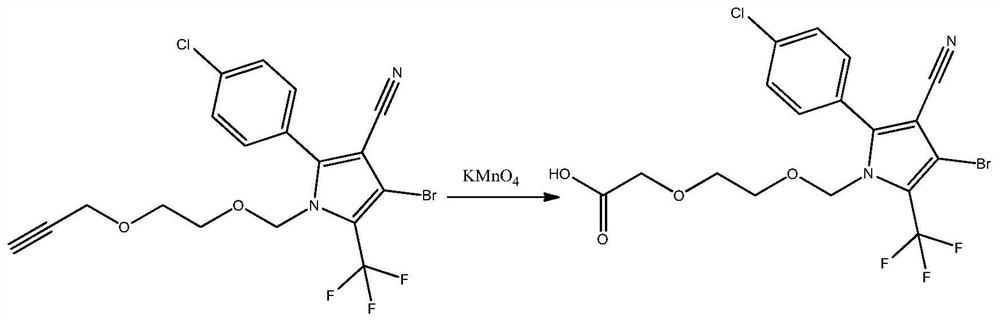
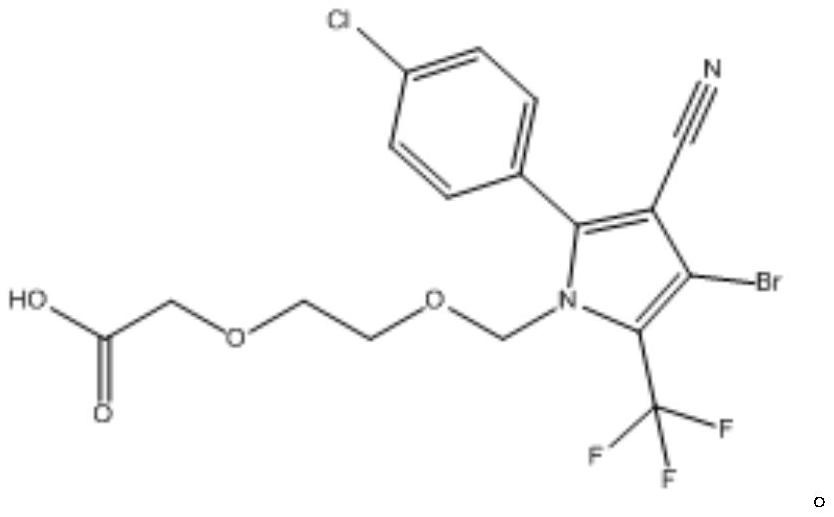
Chlorfenapyr hapten, chlorfenapyr artificial antigen and chlorfenapyr antibody as well as preparation method and application thereof
An artificial antigen, chlorfenapyr technology, applied in chemical instruments and methods, animal/human proteins, serum albumin, etc., can solve the problem of increased identification difficulty, reduced antibody affinity and specificity, and unfavorable hapten molecular characteristics. structure exposure and other problems, to achieve the effects of high purity and yield, enhanced immunogenicity, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of chlorfenapyr hapten, the steps are as follows:
[0033] Take 0.46g of alkynyl chlorfenapyr and add 70mL of 1mol / L dilute hydrochloric acid to dissolve, add 0.16g of potassium permanganate while stirring, heat after stirring well, react at 60°C for 2h, after the reaction is completed, add 6mol / L NaOH solution to adjust the pH to 6 , add ethyl acetate 80mL for extraction, separate the water phase, add 60mL of water to the organic phase and wash once, separate the water phase, add anhydrous sodium sulfate to dry and evaporate to dryness, purify with a silica gel column, and use dichloromethane and methanol at a volume ratio of 10 : 1 mixed solvent elution separation to obtain chlorfenapyr hapten 0.37g.
Embodiment 2
[0035] A preparation method of chlorfenapyr artificial antigen, the steps are as follows:
[0036] Take 18 mg of the chlorfenapyr hapten prepared in Example 1, add 1 mL of tetrahydrofuran to dissolve, add 14.2 mg of N-hydroxysuccinimide (NHS) and 17.4 mg of carbodiimide (EDC), and react at room temperature for 3 hours to obtain a hapten solution Liquid A: take 50 mg of human serum albumin (HSA), add 0.05mol / L PB buffer to dissolve, and obtain liquid B; add liquid A dropwise to liquid B, react at 4°C for 12 hours, and dialyze and purify with 0.02mol / L PBS For 3 days, the medium was changed 3 times a day to obtain the chlorfenapyr artificial antigen coupled with human serum albumin, which was aliquoted and stored at -20°C.
Embodiment 3
[0038] A preparation method of chlorfenapyr artificial antigen, the steps are as follows:
[0039] Take 9 mg of the chlorfenapyr hapten prepared in Example 1, add 1 mL of N,N-dimethylformamide (DMF) to dissolve, add 7.8 mg of NHS and 9.7 mg of EDC, and react at room temperature for 3 hours to obtain hapten solution A; Ovalbumin (OVA) 50mg, add 0.05mol / LPB buffer solution to dissolve to obtain solution B; add solution A to solution B dropwise, react at 4°C for 12h, dialyze and purify with 0.02mol / L PBS for 3 days, change the solution every day Three times, the chlorfenapyr artificial antigen coupled with ovalbumin was obtained, aliquoted, and stored at -20°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com